Abstract
The endoscopic endonasal approach has become the preferred surgical approach to many tumors of the ventral skull base, both benign and malignant. This approach represents an amalgamation of various surgical techniques that have allowed the concepts of minimally invasive surgery to be applied to ventral skull base surgery. Radiologists play an important role in the multidisciplinary skull base team and provide important diagnostic information required for surgical planning, postoperative management and follow-up. This chapter aims to familiarize radiologists with the endoscopic endonasal skull base surgical technique, its history, and what the surgeon wants to know. A variety of illustrative examples are used to emphasize key features and important concepts.
Keywords
Complications of endoscopic skull base surgery, Endoscopic endonasal skull base surgery, Postoperative skull base imaging, Preoperative skull base imaging, Skull base imaging, Skull base surgery
Introduction
The endoscopic endonasal approach has become the preferred surgical approach to many tumors of the ventral skull base, both benign and malignant. Although advances in surgical equipment and technique have played a role in the development, imaging remains one of the cornerstones to the endoscopic endonasal approach, playing an important role in the diagnosis from the preoperative planning stage to intraoperative navigation, postoperative complications, and beyond.
This chapter aims to familiarize radiologists with endoscopic endonasal skull base surgery, including an introduction to the surgical approach, preoperative imaging assessment, postoperative imaging, and follow-up.
Historical Perspective
In many ways, the endoscopic endonasal approach represents the confluence and continued evolution of surgical technology, drawing influences from a variety of surgical approaches: the transsphenoidal approach, which has for some time been the preferred approach in pituitary surgery; the open craniofacial approach, which has traditionally been the preferred approach to tumors of the ventral skull base; and endoscopic sinus surgery, which opened the door to the endonasal corridor.
The transsphenoidal approach dates back more than a century. Schloffer is generally regarded as the father of modern transsphenoidal surgery, having performed the first transsphenoidal resection of a pituitary tumor in 1907. A number of others, including Oskar Hirsch, Harvey Cushing, and Norman Dott, used the technique; however, it was the French neurosurgeon Gerard Guiot who popularized the technique as the preferred approach to the pituitary in 1956. Jules Hardy, a student of Guiot, revolutionized the approach with the introduction of the operating microscope and microsurgical instrumentation in 1967.
The history of anterior skull base surgery dates back to the 1940s, and the first transcranial excisions of orbital tumors were performed by Dandy and Ray and Mclean. In the 1950s and 1960s, the anterior craniofacial approach emerged as the preferred approach for the resection of anterior skull base tumors after it was popularized by Ketcham et al. in 1963. Since then, open craniofacial surgery has been the gold standard for surgical management of most tumors of the ventral skull base.
The history of endoscopic sinus surgery dates back to the 1970s, when an Austrian otolaryngologist, Messeklinger, and his protégé, Stammberger, popularized the operation as a treatment for chronic rhinosinusitis. In 1996, McCutcheon et al. began using the endoscope to assist in the removal of the extracranial portions of anterior skull base tumors. Thaler et al. expanded on this in 1999, pointing out potential indications for and advantages of using an endoscopic-assisted approach to anterior craniofacial resections. By 2001, the first entirely endoscopic endonasal anterior skull base resections had been described by Casiano et al. for the resection of esthesioneuroblastomas. Since then, the endoscope has become an essential tool in the armamentarium of the skull base surgeon, and its use has extended well beyond the sellar region to tumors primarily arising from the anterior cranial fossa, parasellar region, parts of the middle cranial fossa, paraclival region, and craniovertebral junction.
Preoperative Planning
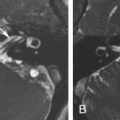
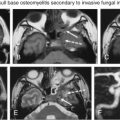
All patients planned for endoscopic skull base surgery should undergo complete clinical and radiologic evaluations when decision is made whether or not a certain tumor is suitable for excision via an endoscopic endonasal approach. MRI and CT are often used in concert, providing information that is complementary. For example, CT performs better for the assessment of the bony involvement and anatomic features, such as the extent of pneumatization, whereas MRI is generally better at resolving soft tissue details. Certain skull base lesions, such as the cholesterol granuloma, fibrous dysplasia, or juvenile angiofibroma, have imaging characteristics that can be diagnosed before biopsy, potentially expediting further management decisions. Radiologic evaluation before biopsy is also useful for identifying situations where biopsy is contraindicated, such as in highly vascular lesions or potential “do not touch” lesions, such as arrested pneumatization, aneurysm, or meningoencephalocele ( Fig. 4.1 ). The imaging is also integral to the actual surgery, as volumetric MRI and CT datasets are typically used for intraoperative navigation.

Multislice CT scanners allow the acquisition of high-resolution, thin-slice images within seconds. These images can be viewed as multiplanar and three-dimensional (3D) reformations for preoperative planning and intraoperative navigation. Reconstruction using a bone algorithm allows accurate assessment of the bony architecture. The presence of bony remodeling or bony invasion can help to differentiate an expanding lesion from an invasive process ( Fig. 4.2 ). CT is superior to MR in recognizing important bony anatomic variants that have surgical implications, such as the degree of pneumatization or hyperostosis of the sphenoid sinus, osseous course of the ethmoidal arteries below the skull base, dehiscence or variant septal attachment along the carotid canal, and bony impressions of the internal carotid arteries (ICAs), as well as any dehiscence or variant pneumatization along the optic nerve canals (Onodi cells). Other surgical landmarks that may be useful include identification of any dehiscence of the lamina papyracea, the height of the lateral lamella or depth of the olfactory fossa, and any asymmetry of the skull base, particularly the cribriform plates and fovea ethmoidalis.

High-resolution pre- and post-gadolinium-enhanced MRI sequences are useful for lesion localization, determination of the degree of orbital involvement; assessment of skull base, dural, and intracranial extension; and planning of the surgical approach. A high tissue contrast allows identification of critical neurovascular structures, including the cranial nerves (particularly the optic nerves, chiasm, and tracts), ICAs, circle of Willis, and cavernous sinus in relation to the lesion.
Depending on the nature of the lesion, tumor extent is usually best assessed on contrast-enhanced T1-weighted (T1W) sequences. Tumor enhancement is typically less than that of normal mucosa and can be recognized as an intermediate shade of gray on contrast-enhanced sequences (affectionately known as the “evil gray”). The pattern of the enhancement of a mass allows the differentiation of tumor from normal mucoperiosteum, inflammatory polyps, obstructive or inflammatory secretions, and inspissated debris.
Bony invasion should also be recognized preoperatively. In our institution, high-resolution, non-fat-suppressed spin-echo T1W pre- and post-gadolinium-enhanced sequences are employed to provide a detailed anatomy of the skull base and soft tissues. Replacement of the normal hyperintense fat signal in the bone marrow often serves as early evidence of disease involvement, and the enhancement pattern can help the clinician to distinguish between edema and tumor infiltration.
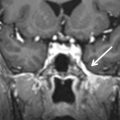
The periorbita is one of the key landmarks in preoperative planning. Not only does the fibrous periorbita represent a relative barrier to the spread of tumor, but it also, when breached, can have grave implications in terms of resectability, morbidity, and prognosis. The interface between tumor and orbit should be carefully assessed and described in the radiology report. The normal periorbita is seen as a thin, hypointense line when viewed perpendicularly on T1W and T2-weighted (T2W) MRI sequences. It is usually best assessed on T2W sequences. Some tumors displace the periorbita without invading it, and these tumors may be safely removed without the need for orbital exenteration. Therefore, it is important to determine whether the periorbita is truly breached or simply displaced. On imaging, the periorbita is considered intact if the linear hypointense signal of the periorbita remains visible at the interface between tumor and orbital fat ( Fig. 4.3 ). The displaced but intact periorbita is typically bowed into the orbit but tapers smoothly toward the point at which it remains attached to the bone. Findings that suggest periorbital invasion include disruption of the hypointense linear signal ( Fig. 4.4 ) or an irregular interface between the tumor and the orbit, with infiltration of the orbital fat ( Fig. 4.5 ). In some cases, particularly at curved or oblique surfaces, doubt will remain. Standard axial and coronal planes may be combined with carefully planned oblique or 3D sequences when a particular interface is in question.



Another important imaging feature is the extent of intracranial involvement. Sagittal and coronal sequences are generally more useful than axial sequences when assessing intracranial extension in the anterior cranial fossa. CT is useful to determine the extent of bony involvement. Bony erosion is often seen as a sign of periosteal invasion. Again, it is important to distinguish aggressive patterns of bone erosion from more indolent remodeling and demineralization, where the periosteum may remain intact. MRI is the modality of choice for the assessment of intracranial involvement. To enter the intracranial compartment, tumor must first cross the bone and its double-layered periosteum. From there, tumor must then cross the dura mater and subarachnoid space to reach the pial surface of the brain. The normal dura is seen as a thin, enhancing layer on postgadolinium T1W images, whereas the cerebrospinal fluid (CSF) signal in the subarachnoid space is best assessed on T2W sequences. The bone-periosteum complex is seen as a thin hypointense linear structure on T1W and T2W sequences. When this remains intact, a tumor is considered extracranial. Smooth, intact, linear dural thickening and enhancement, less than 5 mm thick, is typically associated with reactive dural changes and is not considered as an indicator of dural invasion. When present, this finding suggests a tumor is intracranial but extradural. If the dural thickening is more focal and nodular and shows the same enhancement pattern as tumor (often an intermediate “evil gray” signal), then the lesion is considered intracranial and intradural ( Fig. 4.16 ). The presence of intradural invasion influences the extent of dural resection and affects the size and complexity of the skull base reconstruction. The extent of pial or brain parenchymal involvement, as demonstrated by enhancement extending along the surface of the brain; vasogenic edema; or frank parenchymal invasion also determine the lesion’s resectability.
Perineural spread of tumor is another important feature to recognize. It should be mentioned that when perineural spread is reported radiologically, it refers to the macroscopic spread of tumor away from the primary site of disease, rather than the microscopic finding of perineural infiltration seen on histologic specimens. In these cases, findings that are suggestive of perineural spread include enlargement and enhancement of major nerves, infiltration of extracranial fat pads, foraminal enlargement, and secondary signs associated with denervation. In our institution, high-spatial-resolution, non-fat-suppressed spin-echo T1W pre- and post-gadolinium-enhanced sequences are employed to provide a detailed anatomy of the skull base and soft tissues without the problem of susceptibility artifacts associated with the use of frequency-selective fat-suppression techniques. An alternative approach is to use pre- and postcontrast isotropic high-spatial-resolution volumetric interpolated breath-hold sequences with fat saturation to produce high-spatial-resolution images around the skull base without artifacts.
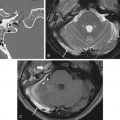
In addition to standard multiplanar MRI sequences, MR angiogram and CT angiogram may be useful for identifying and assessing the patency of the ICA and the anatomy of the circle of Willis in relation to the tumor. For example, the retropharyngeal course of the ICA is an important surgical consideration in the transodontoid approach for lesions in the craniocervical region and a medial course of the cavernous carotids may narrow the surgical corridor when considering a transsphenoidal approach ( Fig. 4.6 ).

Surgical Approaches and Anatomic Limitations
Until recently, open surgical approaches dominated as the preferred surgical approach for tumors involving the anterior skull base, including those arising from the sinonasal cavity. Better understanding of endonasal anatomy, technologic advances in surgical equipment such as high-definition camera systems and computer-assisted neuronavigation, and the establishment of multidisciplinary skull base teams have allowed the endonasal approach to become a safe and viable alternative for major anterior skull base surgery. The concept behind the minimally invasive technique is to provide “access and visualization through the narrowest practical corridor,” allowing for “maximum effective action at the target with minimal disruption of normal tissues.” Endoscopic endonasal approaches use the nasal cavity as a surgical corridor to approach anterior skull base lesions, which provides the most direct and least traumatic surgical option while maintaining an acceptable complication rate, of which CSF leak remains the main risk. It is less invasive than open craniofacial surgery and obviates the need for brain retraction while reducing the risk of damage to critical neurovascular structures that would ordinarily be traversed during open surgery.
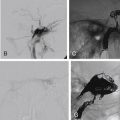
Various classifications systems have been used to define these approaches. Kassam et al. popularized an anatomically based modular approach to endoscopic endonasal surgery, defining the approach in terms of the “sagittal plane” modules ( Fig. 4.7A ), roughly limited laterally by the orbits and ICAs, and the “coronal plane” modules ( Fig. 4.7B ), which describe the lateral limits of the endoscopic endonasal approach. A simplified overview of these approaches is provided in the following sections.

Sagittal plane
Transcribriform
Typical disorders in the anterior cranial fossa accessed via the transcribiform approach include sinonasal tumors such as esthesioneuroblastoma and olfactory groove meningioma.
The endoscopic endonasal surgical access typically involves septectomy, bilateral complete ethmoidectomies, and bilateral frontal sinusotomies with removal of intersinus septum (modified Lothrop or Draf 3). This can be extended laterally with maxillary antrostomies and resection of the lamina papyracea if necessary.
Contraindications to a purely endoscopic endonasal resection include periorbital invasion, superolateral extension of the tumor beyond the mid orbit, invasion through the nasal bone or anterior wall of the frontal sinus, or involvement of the subcutaneous tissues or the skin.
The reporting radiologist should report the presence and extent of intracranial extension and leptomeningeal as well as any cerebral invasion to allow the surgeon to plan the approach with the highest possibility of a complete resection.
Transplanum
The transplanum approach to the suprasellar cistern and third ventricle allows the surgeon direct access without having to displace the optic nerves or carotid arteries. It is typically performed as an extension of a transsellar approach.
Typical lesions amenable to the transplanum approach include pituitary adenomas with suprasellar extension or extension into the third ventricle, preinfundibular portion of craniopharyngiomas not extending lateral to the carotid arteries, Rathke cleft cyst, clival chordomas with suprasellar extension, and planum sphenoidale meningiomas.
The endoscopic endonasal surgical access typically involves bilateral posterior ethmoidectomy, posterior septectomy, and bilateral sphenoidectomies.
Relative contraindications that may preclude complete tumor resection include vascular encasement of the anterior cerebral artery, cavernous sinus invasion, extension lateral or posterior to the carotid arteries, and extension into the orbital apex.
Transsellar
The transsellar approach allows access to sellar lesions, such as pituitary adenomas and Rathke cleft cysts (this may be considered as a suprasellar approach), and is combined with the transplant approach for suprasellar lesions, such as craniopharyngiomas and tuberculum sellae meningiomas. The medial cavernous sinus is also accessed through the transellar approach.
Endoscopic endonasal surgical access typically involves posterior ethmoidectomies, posterior septectomy and bilateral sphenoidectomies, and possible creation of a nasoseptal flap.
Tumor extension lateral to the supraclinoid ICAs or invasion of the lateral cavernous sinus is a contraindication for a pure endoscopic approach.
Transclival
The clivus is divided into the upper, middle, and lower thirds anatomically by the neural foramina. The upper third extends from the posterior clinoids to the Dorello canal (containing the abducens nerve, cranial nerve [CN] VI). Endonasally, the middle clivus lies between the floor of the sella and the floor of the sphenoid sinus, whereas the lower clivus extends from the floor of the sphenoid sinus to the foramen magnum.
The upper transclival approach is seldom used. It requires pituitary transposition to access the interpeduncular cistern, basilar apex, and the posterior third ventricle. The pituitary gland is mobilized onto the planum sphenoidale and the posterior clinoid removed, allowing access to lesions with retroinfundibular extension such as craniopharyngiomas.
The middle transclival approach allows access to the ventral pons, prepontine cistern, basilar trunk, and cisternal portion of CN VI.
The lower transclival approach allows access to the premedullary cistern, vertebrobasilar junction, vertebral arteries, posterior inferior cerebellar arteries, and the cisternal portion of CN IX to XII.
The lateral (transpterygoid) extension of the transclival approach allows access to the jugular fossa.
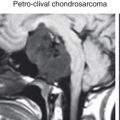
Typical tumors amenable to the transclival approach include clival chordomas, chondrosarcomas, and petroclival meningiomas.
Transodontoid
The transodontoid approach allows access to the inferior clivus, foramen magnum, and C1-C2 vertebrae.
Endoscopically, this extends from the roof of the nasopharynx to the level of the hard palate (corresponding to the level of the C2 vertebra). The fossa of Rosenmüller, with the parapharyngeal ICA laterally, marks the lateral limit.
Typical disorders accessed through a transodontoid approach include inferior clival chordoma, chondrosarcoma, and foramen magnum or craniocervical junction meningiomas.
The extension of surgery laterally in the coronal plane requires a sound understanding of the course of the parapharyngeal ICA from the neck into the petrous canal. The ICA must be identified, and this often requires exposure of the maxillary sinus, a transpterygoid approach with mobilization of the pterygopalatine contents and Eustachian tube laterally.
Coronal plane
The coronal modules are complex and can be simplified into transorbital, medial petrous, suprapetrous, and infrapetrous modules. The suprapetrous and infrapetrous modules are accessed via a common transpterygoid approach. It is also important to remember that the coronal plane refers to paramedian access at different depths, moving from superior to inferior in the ventral to dorsal direction, corresponding to the levels of the anterior fossa and orbits, middle fossa and temporal lobe, and the posterior fossa.
Transorbital (orbital apex/superior orbital fissure/orbit)
The transorbital approach allows access to orbital lesions, such as orbital hemangioma, schwannoma, and meningioma.
Endonasal access of the orbits is through the lamina papyracea after ethmoidectomy. Care should be taken to avoid the optic nerves and the intraorbital vasculature.
Medial petrous
The medial petrous apex is accessed via the medial petrous approach. Typical disorders involving the petrous apex include cholesterol granulomas, whereas petroclival lesions include meningioma, chondrosarcoma, and chordoma, generally involving paramedian extension from the transclival approach.
Transpterygoid (suprapetrous and infrapetrous modules)
The suprapetrous and infrapetrous modules are both accessed by a common transpterygoid approach.
The transpterygoid approach allows access to lesions that may involve the lateral recess of the sphenoid sinus. The transpterygoid-suprapetrous approach accesses Meckel cave, the lateral compartment of the cavernous sinus (sometimes considered as a separate zone), and the middle cranial fossa. Examples of tumors involving the lateral cavernous sinus include invasive pituitary adenoma, whereas pathologies in the Meckel cave include schwannoma and meningioma.
The transpterygoid-infrapetrous approach gives access to lesions in the infratemporal fossa and parapharyngeal space. Examples of infrapetrous disorders include lateral extension of chondrosarcoma, cholesterol granuloma, cholesteatoma, and chordoma. Disorders in the infratemporal fossa and parapharyngeal space include schwannoma, meningioma, angiofibroma, and CSF leak.
A cavernous ICA aneurysm is an absolute contraindication to endoscopic endonasal surgery. Other contraindications include lesions involving the superolateral region of the cavernous sinus, which contain the cranial nerves.
Preoperative identification of pneumatization of the pterygoid plate, vidian canal, ICA, and optic nerve is important to avoid potential complications.
Contraindications
It is important to be aware of some of the features on preoperative imaging that represent relative contraindications to a purely endoscopic approach ( Box 4.1 ). Some of these features may preclude a purely endoscopic approach or require a combined approach to achieve optimal local disease control.