Fig. 1
Normal distribution of 18F-choline
2.2 11C-Acetate
Acetate is absorbed by cells and converted into acetyl-CoA. In this form, it can be involved into two different metabolic pathways: either anabolic or catabolic. Anabolic means that it can be used to synthesize cholesterol and fatty acids, thus forming cell membrane elements. Catabolic means that it can be oxidized in mitochondria by tricarboxylic acid cycle to CO2 and H2O, thus producing energy. Liu (2006) suggested that fatty acid metabolism, more than glycolysis, may be increased in PCa cells. Preclinical studies suggest an extensive involvement of the fatty acids synthesis pathway in acetate uptake in PCa and the upregulation of the key enzyme fatty acid synthase may play a role in genesis of prostate carcinomas (Vavere et al. 2008; Pflug et al. 2003).
Normal biodistribution of 11C-acetate demonstrates high accumulation in the pancreas, variable uptake in the liver and bowel, and some renal uptake, with little urinary excretion. Therefore, the elimination of 11C-acetate does not interfere with pelvic imaging (Seltzer et al. 2004; Fricke et al. 2003). In general, the biodistribution of 11C-acetate is very similar to 11C-choline. 11C-acetate, as well as the other below-discussed tracers, has been investigated for intra-prostatic primary tumor detection and staging as well as for re-staging of PCa in case of biochemical relapse. As with radiolabeled choline, the use of 11C-acetate for accurate detection of intra-prostatic cancer and the differentiation between cancer and normal prostatic tissue or benign hyperplasia is not feasible (Kato et al. 2002; Castellucci and Jadvar 2012). Kotzerke et al. (2002) found no significant difference between the use of 11C-acetate and 11C-choline in the detection of local recurrence after radical prostatectomy (RP), and Vees et al. (2007) found no significant difference between the detection rate of 11C-acetate and 18F-choline PET/CT. In summary, both 11C-acetate and 11C-choline appear to be equally useful in imaging PCa in individual patients, although more comparative data are eligible. In the era of 18F-choline with its advantage of a relatively long half-life, the potential of being used in centers without on-site cyclotron and at least being commercially available, it remains unclear if these studies will be performed ever. Recently, acetate labeled with a longer lived positron emitter, such as 18F, has been preliminary explored in preclinical studies (Ponde et al. 2007). But 18F-fluoroacetate is not a functional analog of 11C-acetate in normal physiology as it demonstrated prolonged blood retention, rapid clearance from liver, excretion in bile and urine, and high bone uptake due to defluorination (Lindhe et al. 2009). Its potential clinical use in PCa remains to be determined.
2.3 18F-Fluorodeoxyglucose (FDG)
Elevated glucose metabolism in malignant tissue in comparison with the normal tissue is based on increased expression of cellular membrane glucose transporters (Glut-1) and enhanced hexokinase II enzymatic activity in tumors (Gillies et al. 2008; Macheda et al. 2005; Smith 2000). PET-imaging with 18F-FDG, an analog of glucose, tracks the glucose metabolism of tissues. The integral role of FDG PET in oncology has been proven for many different tumors in different clinical situations. However, determination of the exact utility of FDG PET in PCa has not been defined so far and is still evolving (Jadvar 2011). FDG PET/CT showed a sensitivity of 80 % and a positive predictive value of 87 % for detection of prostate tumors with a Gleason score of 7 and greater in men who present with more than an intermediate risk of PCa based on elevated serum PSA level (Minamimoto et al. 2011). It appears that FDG PET may reflect the prostate tumor biology with more accumulation in more aggressive lesions that in less aggressive or indolent lesions (Castellucci and Jadvar 2012). 18F-FDG accumulation may overlap in normal prostate tissue, benign prostatic hyperplasia, and PCa tissues significantly, all of which often coexist (Salminen et al. 2002) and false-positive results may occur with prostatitis (Kao et al. 2008). 18F-FDG PET was less sensitive than 99m Tc-based bone scintigraphy at identifying bone metastases (Shreve et al. 1996). Compared to other tracers 18F-FDG PET/CT seems neither suitable in the diagnosis or loco-regional staging of clinically organ-confined disease nor in the detection of locally recurrent disease because of the relatively similar uptake of 18F-FDG by the post-therapy changes and malignant lesions and because of the high level of excreted radiotracer in the urinary bladder that may mask any lesions in adjacent tissues (Liu et al. 2001). FDG PET/CT may be particularly useful in men with advanced PCa (Fox et al. 2011) as it may distinguish metabolically active osseous lesions from metabolically dormant lesions (Morris et al. 2002). Other studies have also shown a potential prognostic utility for FDG PET with generally higher tumor standardized uptake values (SUV) indicating poorer prognosis than those with lower SUVs, which is similar to the general experience with the other cancer types (Oyama et al. 2002; Meirelles et al. 2010). In summary, although FDG PET/CT is generally limited in the diagnosis and staging of clinically organ-confined disease, it may be able to reflect tumor aggressiveness, potentially detect disease sites in a fraction of men with high serum PSA level at the time of biochemical failure, and be useful in the objective assessment of response to chemotherapy or anti-androgen therapy, and in prognostication (Castellucci and Jadvar 2012).
2.4 18F-Fluoride
18F-Fluoride diffuses through bone capillaries into the bone extracellular fluid. Its plasma clearance is very rapid and its single-passage extraction efficiency is high. The fast blood clearance of 18F-fluoride provides an optimal target to background ratio. 18F-fluoride ions exchange with hydroxyl groups in the hydroxyapatite, at the surface of bone crystals, being particularly active at sites of bone remodeling with high turnover. Therefore, 18F-fluoride uptake represents osteoblastic activity in the neighborhood of osteoblastic, lytic, or marrow-based bone metastases (Jana and Blaufox 2006; Even-Sapir et al. 2007). Recent studies have shown good diagnostic performance of 18F-fluoride, resulting in a sensitivity of 89 % with a specificity of 91 %, but compared to 18F-choline there was no advantage; thus, the specificity of 96 % of 18F-choline was significantly higher with the same sensitivity (Langsteger et al. 2011). Also in the recurrence situation, 18F-fluoride was useful in the detection of occult metastases (Jadvar et al. 2012). Although 18F-fluoride-PET is widely considered superior to classical bone scintigraphy, no prospective studies have yet demonstrated an incremental benefit in staging or patient management. Further experience with 18F-PET/CT is required before it may replace conventional single photon bone scans, which are less expensive and more widely available (Bauman et al. 2012).
2.5 Other Tracers
Other tracers have been or are under current investigation. F18-FACBC (anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) is a synthetic l-leucine analog (Fox et al. 2012) and 11C-methionine is a radiolabeled amino acid. As an essential amino acid, L-methionine plays a central role in the altered metabolism of cancer cells, and the latter has been also studied extensively for brain tumor imaging (Grosu et al. 2005a, b, 2006, 2011); both tracers reflect increasing amino acid transport as a precondition for protein synthesis. 18F-FDHT (18F-fluoro-5α-dihydrotestosterone) tracks androgen receptor expression and reflects binding capacity (Liu et al. 1992); androgen receptors are upregulated in castrate resistant disease. 18F-3′-deoxy-3′-fluorothymidine (18F-FLT) tracks the thymidine salvage pathway of DNA (Bading and Shields 2008). Zr89-DFO-huJ591 is a monoclonal antibody to an epitope on extracellular domain of prostate-specific antigen (PSMA) promising for imaging and immunotherapy purposes (Fox et al. 2012; Pandit-Taskar et al. 2008). These radiotracers are able to visualize specific metabolic pathways or cell receptors. However, their use in the clinical context has not been clarified, thus requiring ongoing and future studies; their potential clinical benefit lies beyond the scope of this article.
3 Hardware and Technical Considerations
Integrated PET/CT imaging based on the intrinsic combination of PET and CT within a combined gantry adjustment results in the acquisition of complementary image information within a single examination protocol without the need to reposition the patient (Townsend 2008). The first PET/CT systems started in 2001, and since then staging and restaging of cancer patients has been improved significantly over stand-alone CT- and PET-data acquisition (Czernin et al. 2007; Thorwarth et al. 2012). Modern PET/CT systems for clinical use combine a whole-body, full ring PET and a multi-slice CT (Thorwarth et al. 2012; Lonsdale and Beyer 2010). Scintillation detectors (typically lutetium oxyorthosilicate (LSO)- or lutetium yttrium oxyorthosilicate (LYSO)-based detectors) are circularly arranged and provide a transverse field-of-view of 60–70 cm with measured isotropic image resolution of around 5 mm, but lesion detectability in PET is not only defined by the spatial resolution of the system, but also by lesion contrast. Thus, lesions that are smaller than the image resolution can still be detected in PET if the contrast between lesion and surrounding tissue is sufficiently high (Thorwarth et al. 2012).
The injected dose depends on the type of radiotracer and is usually in the range of about 3–5 MBq/kg. The uptake phase, the time span after tracer-injection, when the acquisition of the PET-data starts depends on the kind of tracer and on its half-life. Uptake times of 2–120 min are reported in literature (Bauman et al. 2012). For example, delayed imaging after injection of 18F-choline may improve the performance of 18F-choline PET for localizing malignant areas of the prostate, because studies have shown on dual-phase PET of the prostate, areas of malignancy consistently demonstrated stable or increasing 18F-fluorocholine uptake, whereas most areas containing benign tissue demonstrated decreasing uptake (Wurschmidt et al. 2011; Kwee et al. 2006). PET imaging times for a single axial bed position (15–22 cm) are in the range between 1–4 min, resulting in a total emission imaging time for a whole body scan to about 20 min (Thorwarth et al. 2012).
A standard PET/CT examination for whole-body staging contains a CT acquired either for the purpose of attenuation correction (low-dose CT with possibility of anatomical correlation) or acquired for the purpose of full diagnostic information (full dose CT with oral and intravenous contrast-enhancement) followed in any case by a multi-step PET emission scan. It is also possible to acquire both (low dose and full dose multi-phase contrast enhanced CT). Studies have shown that contrast enhanced CT images can also be used for attenuation correction (Thorwarth et al. 2012; Beyer et al. 2004). PET/CT image quality and characteristics depend on the type of examination-protocol, such as patient preparation, administered amount of radiotracer in relation to scan duration, and on the characteristics of the overall system sensitivity, reconstruction method, and settings (Boellaard et al. 2004, 2009). With the new of time-of-flight (TOF) technology and resolution recovery during reconstruction, image quality has been enhanced and consequently the diagnostic quality of the PET images has been improved (Lonsdale and Beyer 2010; Rapisarda et al. 2010; Hoetjes et al. 2010). Consistent and standardized procedures are essential for the expanded use of PET/CT as has been proposed for FDG-PET/CT by the EANM (Boellaard et al. 2010) and are at least desirable for other tracers used in clinical routine. As with the rigorous protocol harmonization efforts by the EANM, every PET/CT center is obliged to ensure high quality assurance to enable best sensitivity and specificity for tumor staging.
4 PET/CT-Imaging of PCa in the primary situation
4.1 Determining T-Stage
PET and PET/CT with 11C- or 18F-labeled choline derivates have been used for detection and determination of local tumor spread at primary PCa diagnosis. Some studies with selected patient groups demonstrated relatively high sensitivities for the detection of primary PCas (Reske et al. 2006; Martorana et al. 2006). Other studies reported lower detection rates. Due to the inability of labeled choline to reliably distinguish between PCa, normal tissue, benign prostatic hypertrophy, or high grade neoplasia, studies showed a high incidence of false-positive findings with consecutively lowered specificity (Farsad et al. 2005; Scher et al. 2007; Giovacchini et al. 2008).
4.1.1 11C-Choline
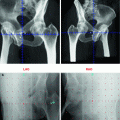
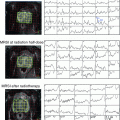
Studies evaluating visualization of primary prostate carcinomas with 11C-choline PET showed considerable overlap of benign, hyperplastic prostate changes, and PCa (de Jong et al. 2002; Sutinen et al. 2004). Farsad et al. examined the usefulness of 11C-choline PET/CT for primary imaging of PCa by correlating imaging findings and histopathologic sextant analysis of axial step sections in 36 patients. All patients underwent RP and pelvic lymph node dissection after 11C-choline PET/CT. A control group consisted of five patients receiving prostatectomy during surgery for bladder cancer. On a sextant basis, histopathology was used to evaluate 11C-choline uptake with respect to PCa, prostatitis, benign prostatic hyperplasia, and high-grade intraepithelial neoplasia (HGPIN). The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of PET/CT were 66, 81, 71, 87, and 55 %, respectively. In the 5 control subjects, high-grade prostate intraepithelial neoplasm was detected at histologic examination in 16 of 30 sextants. PET/CT showed increased 11C-choline uptake in 5 of 16 sextants. This study demonstrated the feasibility of using 11C-choline PET/CT to identify cancer foci within the prostate. However, 11C-choline PET/CT had a relative high rate of false-negative results on a sextant basis and prostatic disorders other than cancer may accumulate 11C-choline (Farsad et al. 2005). Reske et al. investigated 26 patients with clinical stage T1, T2, or T3 and biopsy-proven prostate carcinoma, who underwent 11C-choline PET/CT with subsequent radical retropubic prostatovesiculectomy, and standardized prostate tissue sampling. Maximal standardized uptake values (SUVmax) of 11C-choline within 36 segments of the prostate were determined. PET/CT results were correlated with histopathologic results, prostate-specific antigen (PSA), Gleason score, and pT stage. The SUVmax of 11C-choline in PCa tissue was 3.5 ± 1.3 (mean ± SD) and significantly higher than that in prostate tissue with benign histopathologic lesions (2.0 ± 0.6; P < 0.001 benign histopathology vs. cancer). Visual and quantitative analyses of segmental 11C-choline uptake of each patient unambiguously located PCa in 26 of 26 patients and 25 of 26 patients, respectively. A threshold SUV of 2.65 yielded an area under the receiver-operating-characteristic (ROC) curve of 0.89 ± 0.01 for correctly locating PCa. The maximal 11C-choline SUVmax did not correlate significantly with PSA or Gleason score, but did correlate with T stage (Reske et al. 2006). In 43 patients with known PCa who had received PET/CT before initial biopsy, Martorana et al. assessed sensitivity of PET/CT for localization of nodules 5 mm or greater (those theoretically large enough for visualization) using RP histopathology as the reference standard. PET/CT demonstrated a sensitivity of 83 % for localization of nodular lesions measuring 5 mm or greater. Logistic regression analysis revealed that only size had an influence on sensitivity. For determination of extraprostatic extension sensitivity of PET/CT was low in comparison with MRI (22 vs. 63 %, P < 0.001). The authors concluded PET/CT has good sensitivity for intraprostatic localization of primary PCa nodules 5 mm or greater, but PET/CT does not seem to have any role in staging of extraprostatic extension (Martorana et al. 2006). A study exploring the diagnostic value of 11C-choline PET and PET/CT in a group of 58 patients with suspicion of PCa was conducted by Scher et al. 11C-choline PET and PET/CT demonstrated a sensitivity of 86.5 % and a specificity of 61.9 % in the detection of the primary malignancy. Mean SUVmax for primary malignancy was 4.3 ± 1.7 (2.2–9.8). Mean SUVmax for patients without malignancy was 3.3 ± 0.9 (1.4–4.7) (P = 0.027). The authors concluded that a differentiation between benign and malignant lesions is possible in the majority of cases when image interpretation is primarily based on qualitative characteristics. SUVmax may serve as guidance, but false positive findings may occur due to an overlap of 11C-choline uptake between benign and malignant processes (Scher et al. 2007). Giovacchini et al. (2008) performed 11C-choline PET/CT in 19 patients comparing post-prostatectomy histopathologic sextant analysis axial step sections and 11C-choline PET/CT imaging. Based on a sextant analysis with a 11C-choline SUVmax cutoff of 2.5 PET/CT showed a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 72, 43, 64, 51, and 60 %, respectively. A retrospective study compared the diagnostic performance of MRI, 3-dimensional MR spectroscopy, combined MRI/MR spectroscopy, and 11C-choline PET/CT for intra-prostatic tumor sextant localization, with histology as the standard of reference in 26 men with biopsy-proved PCa. The sensitivity and specificity were 55 and 86 %, respectively, for PET/CT, 54 and 75 %, respectively, for MRI, and 81 and 67 %, respectively, for MR spectroscopy. Therefore, in this study, 11C-choline PET/CT demonstrated a lower sensitivity relative to MR spectroscopy alone or combined with MRI (Testa et al. 2007). Souvatzoglou et al. evaluated the dependency of the sensitivity of 11C-choline PET/CT for detecting and localizing primary PCa on tumor configuration in the histologic specimen in 43 patients who underwent RP. SUVmax values were calculated in each segment and correlated with histopathology. The authors found that small focal tumors (<5 mm) and rind-like tumors were poorly detected on PET, whereas larger and well-defined tumors were detected by PET; additionally, PCa tissue could not be distinguished from benign pathologies in the prostate as PCa-SUVmax was not significantly different from BPH-SUVmax (benign prostate hyperplasia) and prostatitis-SUVmax (Souvatzoglou et al. 2011).
4.1.2 18F-Choline
PET and PET/CT with 18F-labeled choline derivates have been examined for detection of prostate cancer foci and determination of T-stage. Kwee et al. performed studies with 18F-choline at two different time points to evaluate the efficacy of delayed 18F-choline imaging or imaging at two time points for the localization of primary prostate carcinoma (7 and 60 min) in 26 men. Tracer uptake in the prostate on the initial and delayed images was measured on a sextant basis. Prostate biopsy or whole-prostate histologic examination after RP was used to classify a prostate sextant as a dominant malignant region or probable benign region. The mean SUVmax for malignant findings significantly increased from 7.6 to 8.6 between early and delayed acquisition. The mean SUVmax for presumably benign lesions significantly decreased between the initial and the late image (4.8–3.9). The areas under the receiver operating characteristic curves for distinguishing dominant malignant regions from probable benign regions based on initial SUVmax, delayed SUVmax, and retention index were 0.81, 0.92, and 0.93, respectively (Kwee et al. 2006). In a subsequent study of Kwee et al., all 15 patients who underwent PET with 18F-choline prior to RP histopathologic analysis was performed on step-sectioned whole-mounted prostate specimens. The SUVmax corresponding to prostate sextants on PET was measured by region of interest analysis and compared with histopathologic results. Histopathology demonstrated malignant involvement in 61 of 90 prostate sextants. The mean total tumor volume per specimen was 4.9 ml (range 0.01–28.7 ml). Mean SUVmax was 6.0 ± 2.0 in malignant sextants and 3.8 ± 1.4 in benign sextants (p < 0.0001). The area under the receiver operating characteristic curve was 0.82 for sextant detection of malignancy based on SUVmax measurement. Tumor diameter directly correlated with sextant SUVmax in malignant sextants (r = 0.54, p < 0.05). The authors concluded that 18F-choline PET can serve to localize dominant areas of malignancy in patients with PCa. However, PET with 18F-choline may fail to identify sextants with smaller volumes of malignancy (Kwee et al. 2008). The so far largest series, comprising 130 patients has been published by Beheshti et al. In 111/130 patients, RP with extended pelvic lymph node (LN) dissection was performed. Patients were categorized into groups with intermediate (n = 47) or high (n = 83) risk of extracapsular extension on the basis of their Gleason scores and prostate specific antigen levels. Significant correlation was found between sections with the highest 18F-choline uptake and sextants with maximal tumor infiltration (r = 0.68; P = 0.0001) on RP specimens (Beheshti et al. 2010).
4.2 N-Staging
4.2.1 11C-Choline
The first study demonstrating that 11C-choline may be useful for identification of metastatic lymphnodes was published by Kotzerke et al. (2000). They described a per patient sensitivity of 50 % and specificity of 90 % in a series of 23 patients with mixed disease stages.
De Jong et al. prospectively examined 67 consecutive patients with histologically proven PCa with 11C-choline PET. The results of PET were compared with the results of histology of the pelvic lymph nodes and with follow-up data. They reported values of 80, 96, and 93 % for per patient sensitivity, specificity, and accuracy (de Jong et al. 2003). Schiavina et al. included 57 patients in a study with proven PCa and an intermediate or high risk for lymph node metastases. Patients underwent 11C-choline PET/CT prior to prostatectomy and extended pelvic lymph node dissection. Fifteen patients (26 %) had lymphnode metastases, and a total of 41 lymphnode metastases were identified. On a patient analysis, sensitivity, specificity, PPV, NPV, and number of correctly recognized cases at PET/CT were 60.0, 97.6, 90.0, 87.2, and 87.7 %, while on node analysis, these numbers were 41.4, 99.8, 94.4, 97.2, and 97.1 %. The mean diameter (in mm) of the metastatic deposit of true-positive LNs was significantly higher than that of false-negative LNs (9.2 vs. 4.2; p = 0.001) (Schiavina et al. 2008).
4.2.2 18F-Choline
Several authors reported about the performance of 18F-choline in detecting metastatic lymphnodes. Husarik et al. (2008) found a per patient sensitivity for nodal detection of 33 % among 43 patients. Beheshti et al. demonstrated in a series of 130 patients a better performance of 18F-choline PET/CT for detecting nodal involvement, particularly among lymph node metastases greater than or equal to 5 mm in size. A total of 912 lymphnodes had been sampled in these patients receiving RP because of high risk and intermediate risk PCa. The reported per lesion sensitivity, specificity, positive, and negative predictive values were 66, 96, 82, and 92 %, respectively (Beheshti et al. 2010). Poulsen et al. (2012) reported among 210 men with intermediate- and high risk PCa undergoing lymphadenectomy and RP a per patient sensitivity, specificity, negative predictive value and positive predictive value of 73 %, 88 %, 59 % and 93 %, while the corresponding values for LN-based analyses were 56 %, 94 %, 40 %, and 97 %, respectively. Both Beheshti and Poulsen focused on men with intermediate- and high-risk PCa, which may account for their findings of better performance of 18F-choline PET/CT in assessing nodal disease.
4.3 M-Staging
In patients suffering from advanced PCa beside locoregional lymphnode metastases frequently distant metastases occur. Typically osseous metastases do appear in large percentage in advanced disease stages. But also soft tissue metastases in lung and liver may be found (Tuncel et al. 2008).
4.3.1 11C-Choline
Tuncel et al. studied the performance of 11C-choline in 45 patients with advanced PCa. Overall, 295 lesions were detected: PET alone, 178 lesions; diagnostic CT, 221 lesions; PET/CT (low-dose CT), 272 lesions; PET/CT (diagnostic CT), 295 lesions. Two thirds of the lesions were located in the bone; one third in the prostate, lymph nodes, periprostatic tissue and soft tissue (lung, liver). The use of diagnostic CT did not result in a statistically significant difference with respect to lesion localization certainty and lesion characterization. PET-negative and PET/CT-positive lesions were mostly localized in the bone (78 %, 91/117) as were PET-positive and CT-negative lesions (72 %, 53/74). Of the latter, 91 % (48/53) represented bone marrow and 9 % (5/53) cortical involvement. The authors concluded that 11C-choline PET/CT improved assessment of metastastic disease including skeletal manifestations, while 11C-PET/CT changed disease management in 24 % of these 45 patients (Tuncel et al. 2008).
4.3.2 18F-Choline
In a preoperative series Beheshti et al. (2010) detected 43 bone metastases in 13/130 patients. Early bone marrow infiltration was detected with only 18F-choline PET in two patients. 18F-choline PET/CT led to a change in therapy in 15 % of all patients and 20 % of high-risk patients. Another study from the same group of investigators correlated the uptake of 18F-choline in bone metastases with the morphologic changes on CT in 70 men with PCa. The standard of reference was other imaging and clinical follow-up. The overall sensitivity and specificity of 18F-choline for the detection of bone metastases were 79 and 97 %, respectively. Lytic lesions demonstrated higher metabolism than blastic lesions. The authors identified 3 correlative PET/CT patterns for bone metastases: lesions with 18F-choline uptake only, probably representing bone marrow infiltration without morphologic changes on CT; lesions with both 18F-choline uptake and CT morphologic changes; and lesions with no 18F-choline uptake, but displaying dense sclerosis on CT (Hounsfield units >825), probably indicating nonviable tumor (Beheshti et al. 2009).
18F-choline and 18F-fluoride have been compared in the detection of bone metastases. Reported sensitivity, specificity, and accuracy was 81, 93, and 86 % for 18F-fluoride, and 74, 99, and 85 for 18F-choline, respectively. This study revealed that 18F-choline might be superior for early detection (i.e., bone marrow involvement) of metastatic bone disease and that in patients with 18F-choline–negative suggestive sclerotic lesions, 18F-fluoride can be helpful, with the limitation that 18F-fluoride PET could also be negative in highly dense sclerotic lesions, presumably reflecting treated disease (Beheshti et al. 2008). Therefore, metabolic and morphologic changes of bone metastases are dynamic processes, and combined imaging is best suited to capture the natural course of these changes to allow for management decisions and accurate assessment of treatment response (Jadvar 2011).
5 PET/CT-Imaging of Recurrent PCa
RP and radiotherapy (RT) are the standard treatment options for clinically localized PCa (Lu-Yao and Yao 1997). However, relapses after initial treatment of localized PCa are not uncommon: the reported rates of biochemical relapse after radical prostatectomy (RP) range from 20 to 53 %, most of them (95 %) occurring in the first 5 years (Han et al. 2003). The reported data after 3D conformal radiation therapy are similar (Chism et al. 2004). Salvage RT is the mainstay therapy in the setting of biochemical relapse after RP that offers the potential of cure. An increase in serum PSA is the most accurate and early index for detecting cancer recurrence or residual disease after RP (Polascik et al. 1999). One of commonly used definitions has been PSA ≥ 0.2 ng/ml with one subsequent rise. The American Urological Association and the European Association of Urology put forward a guideline that recommended PSA ≥ 0.2 ng/ml with a second confirmatory level of >0.2 ng/ml as the definition of PSA relapse (Choo 2010). After radiation treatment, a rising PSA level 2.0 ng/ml above the nadir value is the most reliable indication of persistent or recurrent disease (Heidenreich et al. 2011). Four different recurrence patterns exist: (1) evidence of only local recurrence in the prostatectomy bed or irradiated prostate gland; (2) evidence of only loco-regional metastases in the pelvic lymph nodes (3) distant metastases (most commonly nodal or osseous), and (4) a combination of local and distant recurrence (Sella et al. 2004).
In patients with biochemical relapse, knowing whether the disease is localized in the prostatectomy bed, irradiated prostate gland, respectively or whether metastasis are present is essential for the treatment planning process. Detection of any distant metastasis obviates the need for local salvage treatment with curative intention. This clinical situation requires a method with a high sensitivity and specificity that allows an early detection of disease localization. In the setting of biochemical relapse with rather low PSA levels, conventional radiological tools, such as ultrasound, including transrectal ultrasound in combination with a TRUS-guided biopsy CT and bone scan proved to have only low sensitivities and has therefore no established role in the diagnostic work up in this clinical scenario (Connolly et al. 1996; Deliveliotis et al. 2007; Naya et al. 2005; Saleem et al. 1998; Scattoni et al. 2004; Shekarriz et al. 1999). Modern functional imaging modalities like PET/CT offer excellent additional information to clinical and therapeutic variables and have shown to provide significant impact in patient management and RT-planning that may translate in consecutive improved disease control and survival rates in different tumor entities (Grosu et al. 2006, 2009, 2011; Nestle et al. 2009; Rischke et al. 2012a).
In the situation of biochemical relapse after primary treatment of localized PCa, studies with choline-PET/CT showed promising results to guide management approaches as these imaging modalities can accurately identify the site of recurrence. PET/CT as a whole body examination allows evaluation of local recurrence and distant metastases in a single step. Most of the studies evaluating patients with PSA-recurrence were performed with labeled choline and we focus on both these tracers. Some data were generated using 11C-acetat; however 11C-acetate and 11C-choline appear to be about equally useful in imaging PCa in individual patients (Kotzerke et al. 2003).
5.1 11C-Choline
Many studies using 11C-choline have demonstrated the value of 11C-choline PET or PET/CT in the situation of PSA relapse. For example Picchio et al. compared 11C-choline to FDG for restaging in 100 patients with biochemical recurrence and a mean PSA of 6.6 ng/ml. Areas with pathologic 11C-choline uptake were found in 47 % of patients and in 27 % of patients using FDG-PET. After 1-year follow-up, 80 % of patients who had a negative 11C-choline PET had an unchanged PSA (Picchio et al. 2003). These results also show, that FDG plays virtually no role in the situation of PSA-recurrence. Rinnab et al. examined 50 patients after primary therapy with 11C-choline PET/CT, most of whom were compared to histopathology. The mean (median, range) PSA level in patients with positive PET/CT was 3.62 (2.42, 0.5−13.1) ng/ml, and that in patients with a negative scan was 0.90 (0.95, 0.41–1.40) ng/ml. The sensitivity at a PSA level of <2.5 ng/ml of PET/CT for detecting recurrence was 91 % (95 % confidence interval, 71–99 %) (Rinnab et al. 2007). Reske et al. studied 49 patients who underwent 11C-choline PET/CT after radical prostatectomy, of whom 36 patients had biochemical evidence and histological evaluation of local recurrence. Thirteen patients had PSA <0.3 ng/ml and no evidence of active disease after 1 year follow-up. Mean PSA of 2.0 ng/ml (0.3–12.1) was found in the group of the 36/49 patients with histologically confirmed local recurrence. 11C-choline PET/CT was true positive in 23/33 patients and true negative in 12/13 controls (Reske et al. 2008).
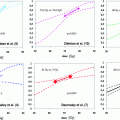
The relationship between serum PSA value and 11C-choline detection rate was first described by Krause et al. They examined 63 patients with biochemical failure after primary therapy (radiation therapy and radical prostatectomy). Within the whole patient group pathologic lesions were seen in 35/63 patients (56 %). Detection rate for PSA values <1 ng/ml was 36 %, 43 % for a PSA-value 1–<2 ng/ml, 62 % for a PSA-value 2–<3 ng/ml, and 73 % for a PSA-value ≥3 ng/ml (Krause et al. 2008). The linear correlation of the 11C-choline detection rate and PSA value was confirmed by Castellucci in a large cohort of 190 patients. In 106/190 patients data were available for calculation of PSA velocity (vel) doubling time (dt). The detection rate was 12, 34, 42, and 70 %, respectively, in patients with PSAvel < 1 ng/ml/year, 1 < PSAvel ≤2 ng/ml/year, 2 < PSAvel ≤ 5 ng/ml/year, and PSAvel >5 ng/ml/year. The 11C-choline PET/CT detection rate was 20, 40, 48, and 60 %, respectively, in patients with PSAdt >6 months, 4 < PSAdt ≤ 6 months, 2 < PSAdt ≤ 4 months, and PSAdt ≤ 2 months. Trigger PSA level and PSA velocity were found to be independent predictive factors for a PET-positive result (P = 0.002; P = 0.04) and PSAdt was found to be an independent factor only in patients with trigger PSA less than 2 ng/ml (P = 0.05) using multivariate analysis (Castellucci et al. 2009).
Another study conducted by Castellucci et al. with 102 patients 11C-choline PET/CT was able to detect recurrent disease in 28 % of the patients with biochemical relapse characterized by low trigger PSA levels (PSA < 1.5 ng/ml). Very interestingly, 11C-choline PET/CT detected distant unexpected metastases in 21 % of the patients. At multivariate statistical analysis only PSA doubling time and nodal status were shown to be significant and independent predictive factors for positive 11C-choline PET/CT. Therefore, 11C-choline could be suggested to be performed early during initial biochemical relapse in patients presenting with fast PSA kinetics (Castellucci et al. 2011).
Giovacchini calculated PSA doubling time retrospectively in 170 patients and demonstrated that the percentage of patients with positive 11C-choline PET/CT was 27 % for PSADT >6 months, 61 % for PSADT between 3 and 6 months, and 81 % for PSADT <3 months. The percentage of patients who displayed pathological 11C-choline uptake in the skeleton significantly increased (p < 0.05) from 3 % for PSADT >6 months to 52 % for PSADT <3 months. Interestingly, patients who displayed pathological 11C-choline uptake in the prostatectomy bed were 0 % for PSADT <3 months and 17 % for PSADT >6 months (p < 0.05) (Giovacchini et al. 2010). An additional analysis of these 170 patients showed that in patients with a positive 11C-choline PET/CT have a significantly higher PSA-velocity (PSAvel) than inpatients with negative scans. The percentage of patients with a positive 11C-choline PET/CT was 21 % for PSAvel less than 1 ng/ml per year, 56 % for PSAvel between 1 and 2 ng/ml per year, and 76 % for PSAvel more than 2 ng/ml per year (Giovacchini et al. 2012). 11C-choline-PET/CT has been shown to be a valuable diagnostic tool for the detection of lymph-node metastases of recurrent PCa. Scattoni et al. evaluated 63 nodal sites in 25 patients (median PSA: 1.98 ng/ml) histologically and described a lesion-based sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 64, 90, 86, 72, and 77 %, respectively. They concluded that the low negative predictive value seems to depend on the limited capability of 11C-choline-PET/CT to detect microscopic lesions, but the high positive predictive value, even with low PSA values, provides a basis for further treatment decisions (Scattoni et al. 2007).
5.2 18F-Choline
Very similar to the published results of 11C-choline, the detection rate obtained by using 18F-choline PET/CT is higher among patients with higher PSA level at the time of recurrence, shorter PSA doubling time, or higher initial Gleason grade. Cimitan et al. described positive 18F-choline scans in 54 patients examined for rising PSA post-radical prostatectomy (n = 58), primary RT (n = 21), or under anti-androgen therapy (n = 21). 18F-choline PET/CT were rarely (3/38) positive among patients with PSA <4 ngml/ml and initial Gleason grade ≤7, whereas all patients with a PSA >4 ngml/ml and Gleason score >7 had positive scans. In patients with PSA <4 ngml/ml and Gleason score >7, 18F-choline PET/CT was positive in 54 % of patients (Cimitan et al. 2006). Pelosi et al. found that PET/CT detected disease relapse in 43 % of cases (24/56). PET sensitivity was closely related to serum PSA levels, showing values of 20, 44, and 82 % in the PSA ≤ 1, 1 < PSA ≤ 5 and PSA >5 ng/ml subgroups, respectively (Pelosi et al. 2008). Husarik et al. (2008) examined 68 patients for restaging at biochemical recurrence. They found a good sensitivity of 83–87 % when PSA was >4 ng/ml. When PSA was <4 ng/ml sensitivity decreased about 70–75 %. A total of 71 patients with biochemical failure were studied by Casamassima et al. after PCa treatment: prostatectomy (n = 28), RT (n = 15), or both (n = 28). Detection of local, pelvic, and extra-pelvic nodal and bone metastases was found in 55 % of patients. Median PSA velocity was 0.40 ng/ml/year in PET-negative patients and 2.88 ng/ml/year in PET-positive subjects (P < 0.05) (Casamassima et al. 2011). In terms of lymphnode recurrence detection similar to Scattoni et al. (2007) the group of Tilki et al. evaluated the accuracy of combined 18F–choline PET/CT in the detection of lymphnode metastases in PCa patients with rising PSA level after radical prostatectomy. The findings of PET/CT were compared with the histologic results. A lesion-based analysis yielded 18F-cholin PET/CT sensitivity, specificity, positive predictive value and negative predictive value of 40, 96, 76, and 83 %, respectively. A site-based analysis yielded sensitivity, specificity, positive predictive value and negative predictive value of 69, 73, 81, and 58 %, respectively (Tilki et al. 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree