Ultrasound Imaging in Renal Transplantation
The number of patients with end-stage renal disease continues to rise and is unlikely to plateau before the middle of this century. This has tremendous implications in terms of public health resources, interms of diagnosis, treatment, and long-term surveillance in an ever increasingly elderly population.1 Prognosis for these patients has generally improved, especially for those with co-morbidity disease processes such as diabetes mellitus.2
A number of treatment options are available, including peritoneal and hemodialysis, but there is no doubt that the optimum treatment option is renal transplantation. There are limitations, namely the continuing shortage of suitable donor kidneys3; however, improvements in outcome due to better donor-recipient matching4 and the use of more potent immunosup-pressive regimens has meant that the one-year and five-year graft survival rates in Europe now stand at 77-82% and 57-64% respectively.5 In order to improve on these figures further, early recognition and appropriate treatment of a variety of transplant insults and complications is required.
Transplantation is undoubtedly the treatment of choice for many patients with chronic renal failure. A well-functioning transplant will provide a glomerular filtration rate (GFR) of 50-60 ml/h, which is sufficient to return most patients to a much more independent life pattern. Leaving aside the significantly improved quality of life, in the harsh world of health economics, the cost benefit of a functioning successful transplant outweighs that of failure and hence many resources are targeted throughout the pre-, peri-, and post-transplant period to ensure high success rates are achieved. Currently the average normal life expectancy of a transplant kidney is 7-10 years, increasing to 15-20 years when a live donor organ is used.
Whilst a number of different modalities are used in imaging the renal transplant, there is no doubt that ultrasound fulfils a very useful role in both the early postoperative period, as a noninvasive indicator of transplant dysfunction, and in the long-term follow-up of these patients. This chapter will highlight the value and application of this technique.
Indications and Contraindications
There are few absolute contraindications to renal transplantation, which should be considered for all patients with end-stage renal disease severe enough to require dialysis. Patients unsuitable for transplantation are generally those who are for whatever reason, generally unfit for anesthesia or surgery, e.g., because of severe arteriopathy or severe cardiac or respiratory disease. In addition, the known hazards of immunosuppression in the context of pre existing infectionormalignancy must be considered, as should the risk of disease recurrence, e.g., in patients with oxalosis or active vasculitis.
Donor Supply
Sources of organ transplantation include cadaveric, brain-dead, ventilated organ donors and live related donors. In developed countries, the majority are cadaveric in origin, whilst live related donor transplantations are favored in developing countries. Although there are some small technical differences in surgical technique and recipient outcome, the overall management of the recipient should be regarded similarly for transplants of either origin.
Histocompatibility Testing
In order to reduce the risk of rejection, especially hy-peracute rejection, the presence of donor-specific antibodies in the lymphocyte cross-match test is a contraindication to transplantation. Graft acceptance in transplant patients with a negative lymphocyte cross-match test depends upon the degree of matching between the donor and the recipient giving lymphocytic antigens (HLA). There are three classes of HLA, all of which are located on chromosome 6.6,7 All are recognized targeted antigens for T cells. HLA status is determined for all donors and recipients at the HLA A, B, and C locations. The importance of this is reflected in the improved graft survival of an HLA-matched graft T1/2 = 17.3 years, compared with an HLA-mismatched graft survival of 7.8 years.8 This improved survival was also demonstrated in another study, where a 17% improvement in five-year graft survival was noted in patients who received matched HLA B and DR kidneys compared to those who did not.9
Preoperative Management
Transplantation should be carried out within 24 hours of organ retrieval if possible, and at worst within 48 hours. During this period, a suitable recipient will have been chosen and appropriately prepared for the operating room, i.e., screened for infection and car-diorespiratory reserve, and additional dialysis given if necessary to correct any fluid or metabolite imbalance.
A live related donor, who may be a family member or a close friend, is screened by means of a thorough history, clinical examination, and HLA status assessment. Tests vary from center to center but will include a 24hour creatinine clearance test, virology, liver function tests, and the choice of intravenous urogram, DMSA scan, arteriography, or computed tomography and/or magnetic resonance imaging to assess the kidneys.
Surgery
The standard method of surgery is a retroperitoneal iliac approach in which the transplant renal vessels are anastomosed to the external iliac vessels in the case of a cadaveric transplant and the internal iliac vessels in the case of a live related transplant. In patients receiving a third transplant, an intraperitoneal approach to the iliac vasculature is often used. The vesicoureteric anastomosis is performed by attaching the shortened donor ureter to the dome of the bladder. As a result of this procedure, the lower end of the ureter is prone to ischemic insult and secondary stricture formation, which may be functionally significant. (For more surgical details, see Chapter 3.)
Postoperative complications vary from center to center but include bleeding (less than 1%), major vascular occlusion of the artery or vein (1-2%), wound infection (1.6-6.3%), and urological complications, including anastomotic leak, obstruction, and hematuria (1.3-7%).10-13
Immunosuppression
The underpinning aim of immunosuppression is to prevent rejection without infectious complications or serious drug toxicity. A number of treatment options are now available and these include regimes of drugs such as cyclosporine (cyclosporin A) or tacrolimus14 and steroids with either azathioprine or mycopheno-late.15 Antibody therapy with anti-interleukin-2 agents is effective and less toxic than with the older humoral agents including OKT3 and antithymocyte globulin. Newer developments, including rapamycin as a replacement for azathioprine or mycophenolate, are currently the subject of clinical trials. Standard immuno-suppressive regimes are variable; however, our localregimen includes a combination of prednisolone, my-cophenolate, and cyclosporine.
The treatment for established acute rejection is normally high-dose oral or intravenous steroids and, in more difficult cases, immunodepletion with antibody therapy or tacrolimus. There is no effective treatment for hyperacute or chronic rejection.
 Summary points:
Summary points:
- The one-year and five-year graft survival rates are now 77-82% and 57-64%
- Contraindications to transplantation are few
- The risk of disease recurrence after transplantation is high in patients with oxalosis or active vasculitis
- Negative HLA cross-matching is essential for a favorable outcome
Imaging the Transplant Kidney
B Mode Ultrasound
The transplant kidney can normally be easily visualized in either iliac fossa a number of centimeters or so beneath the skin surface. Although its orientation may be variable due to the surgical technique, it is normally possible to obtain views of the transplant kidney that are perpendicular to each other. As for any other ultrasound technique, image resolution will depend on a number of factors, including patient build, the depth of the transplant below the skin surface and the presence or absence of postoperative edema. Generally a 4-MHz probe will give a good overall gray-scale assessment of the transplant kidney and is best suited to the detection of peritransplant collections and the assessment of the iliac and transplant vasculature. A higher-frequency probe, i.e., 7 MHz, will give excellent near-field resolution and good anatomical detail of the kidney, but except in the thinnest of patients, will not allow visualization of the deeper-situated vascular structures.
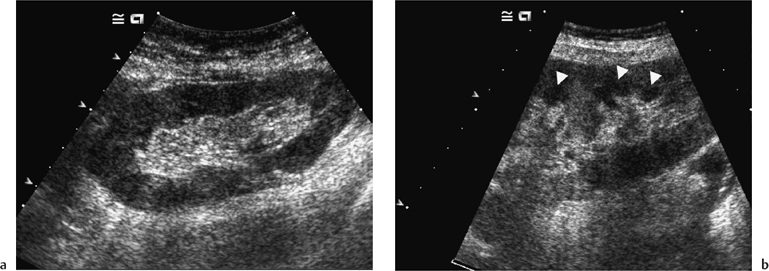
Morphologically the transplant kidney is very similar to the native; any apparent differences being attributable to the better ultrasonic resolution in the former. In essence, there is a well-defined renal parenchyma peripherally with a bright echogenic sinus centrally (Fig. 4.1a). The renal pyramids are more commonly visualized in the transplant kidney than in the native, are hypoechoic relative to the parenchyma, and bear a constant relationship to the medulla, being regularly spaced with no communication between (Fig. 4.1b), this being a useful differential feature from dilated calyces. Mild hydronephrosis maybe visualized with the “normal” transplant kidney, this being most common in the early transplant period when postoperative edema at the lower end of the vesicoureteric anastomosis may be responsible. This may resolve with time. Assuming renal function to be normal, any minor degree of hydronephrosis is normally documented to act as a baseline scan against which to evaluate subsequent studies (Fig. 4.2).
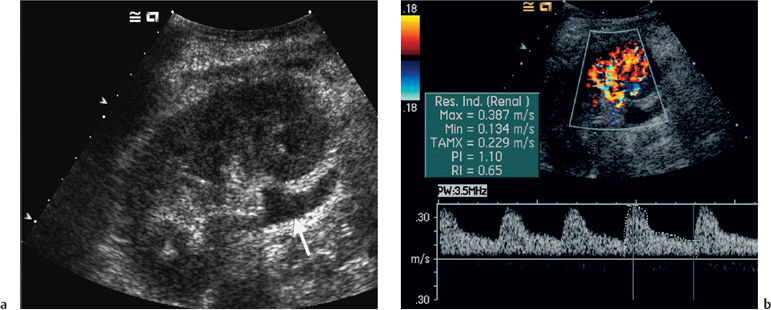
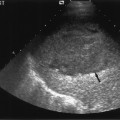
Fig. 4.2 a Ultrasound image of a 5-day-old live related renal transplant showing mild hydronephrosis with some dilatation of the proximal ureter (arrow). b Color Doppler image of the same patient showed excellent renal perfusion and a normal intrarenal spectral Doppler waveform with a pulsatility index (PI) of 1.10. The transplant was functioning normally and therefore the hydronephrosis was simply noted and followed on subsequent studies
The transplant vessels at the renal hilum should not be confused with a slightly dilated or prominent renal pelvis, and can easily be differentiated with the use of color or power Doppler ultrasound. The iliac vessels are normally identified with the color Doppler technique, although spectral Doppler is required for quantitative information. The bladder should be routinely visualized when possible and should be echo free. Ifturbid echoes are present, this may indicate either infection or hemorrhage, depending on the clinical setting.
Color Doppler Ultrasound
Color Doppler imaging provides an instantaneous assessment of the intrarenal vasculature and a global impression of transplant perfusion, and also identifies the transplant artery, vein, and iliac vessels (Fig. 4.3). Such information is purely qualitative and spectral Doppler is required for quantification. This technique is similar to that used elsewhere, i.e., a suitable intrarenal vessel is identified with color flow (normally an interlobar artery), the spectral gate is placed over the vessel, and a Doppler tracing is obtained. The normal Doppler tracing is a low-resistance waveform similar to that seen in the native kidney and hepatic artery. It has previously been described as having a “ski-slope” appearance with diastolic flow normally contributing up to a third or a half of the peak systolic value. Any reduction in end-diastolic flow may reflect a pathological process. For color Doppler ultrasound to be used effectively, sequential studies are required. It is generally accepted that the value of a single isolated examination is limited, and our practice is to perform transplant studies every second day in the early transplant period until renal function is satisfactory.
Many different indices have been used in an attempt to quantify “flow.” These include pulsatility index (PI), resistive index (RI), systolic-diastolic ratio (SDR), and di-astolic-systolic ratio (DSR). The most commonly used indices are the PI and RI; there is no specific advantage of one over the other (Fig. 4.4a).
With regard to the transplant artery, this can be a difficult vessel to study as it is extremely tortuous. As with other Doppler studies, an angle of less than 60° is required for accurate velocity determination, and within the transplant artery a range of normal peak systolic velocity values up to 2.5 m/s have been published.16– At our center the cut-off value is 2.5 m/s; values below this level are regarded as normal (Fig. 4.4b) whilst those above are taken to represent a significant stenosis. The numerous twists and turns of the vessel in combination with a requirement for precise angle correction and thus velocity readings make this a meticulous and demanding examination.
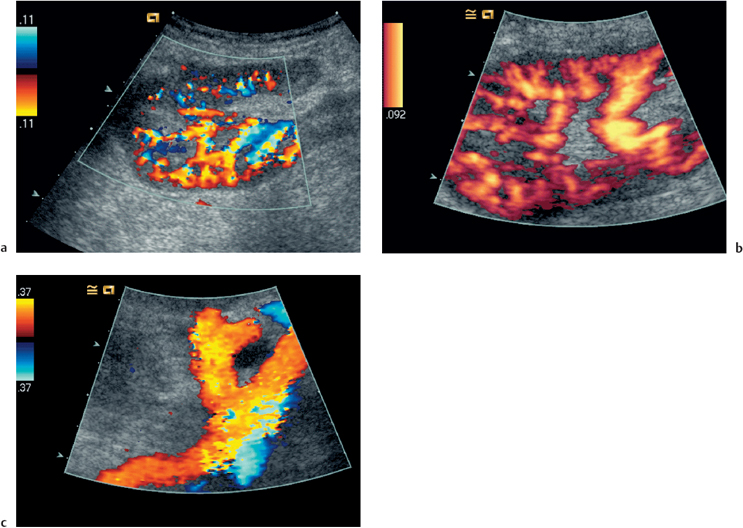
Fig. 4.3 a Color Doppler image showing normal perfusion of the transplant kidney. b Power Doppler scan of a. This technique is more sensitive to flow detection, but all information with regard to flow direction is sacrificed. c Color flow image demonstration of the origin of the transplant renal artery (thick arrow) arising from the external iliac artery (thin arrow)
There are no specific velocity values for flow in the transplant renal vein; however, often the prime consideration is simply the presence or absence of flow. It is always of paramount importance to identify both the iliac artery (Fig. 4.4c) and the iliac vein in order to distinguish these from the renal vessels, and to exclude a more proximal lesion, e.g. a stenosis in the iliac artery, which may contribute to or be the cause of adverse renal function.
 Summary points:
Summary points:
- A 4-MHz ultrasound probe gives the best overall assessment of the transplant kidney
- Mild hydronephrosis is “normal” in the early postoperative transplant period
- Serial measurements with color Doppler ultrasonography can monitor transplant dysfunction
- Both the transplant and the iliac vessels should always be identified to avoid confusion
- The pulsatility index (PI) and resistive index (RI) are the most commonly used Doppler indices
Early Complications
A number of complications are recognized in the early transplant period; these include parenchymal insults, i.e., acute tubular necrosis (ATN), acute rejection, or both; vascular occlusions; obstruction; hemorrhage; urinary leak; collections; infection; and drug toxicity related to the antirejection treatment itself. Many of these complications can be differentiated on the basis of a combination of clinical history, bacteriology. and ultrasound. Very often, however, the main differential diagnosis is between acute rejection and ATN, and this may be difficult clinically as symptoms are often absent. Since the two entities require different treatment, early accurate diagnosis is essential. It is not possible to distinguish between these pathological entities with color Doppler ultrasonography—histology is required. Nevertheless, ultrasound is useful in its dual role of not only monitoring transplant dysfunction but also assessing response to therapy.
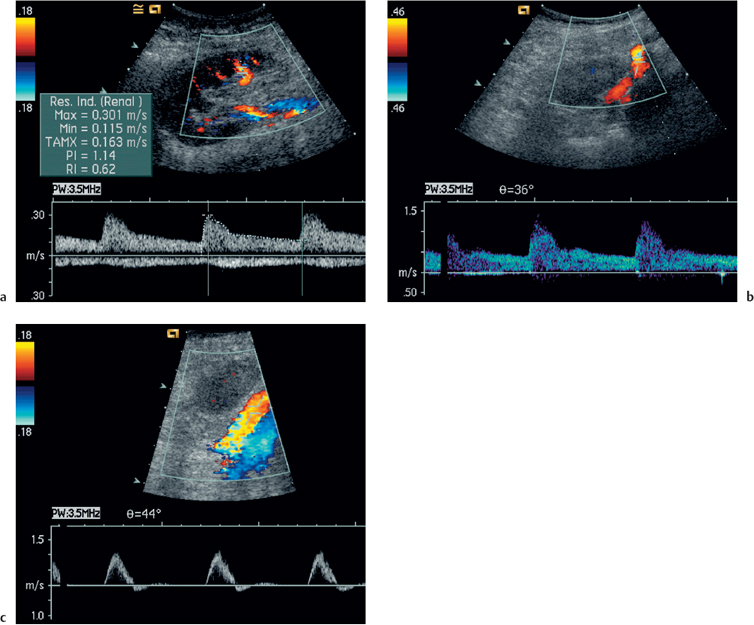
Fig. 4.4 a Normal intrarenal spectral Doppler waveform. There is normal flow throughout diastole, this being reflected in the PI of 1.14 (RI 0.62). The PI is derived by the formula
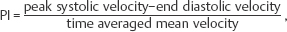
whilst the resistive index
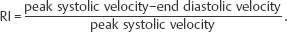
b Normal Doppler waveform from the origin of the transplant renal artery. The peak systolic velocity is 1.0 m/s. c Normal spectral Doppler waveform from the iliac artery just proximal to the origin of the transplant artery. The waveform is triphasic and the peak systolic velocity is 1.0 m/s
Acute Tubular Necrosis
ATN is common in the early transplant period; as many as 10-30 % of patients require dialysis in the early stages. Delayed graft function, rare when a live related donor is used, is principally related to both the donor and the donor kidney, particularly the warm ischemic time. In patients with established ATN requiring dialysis, recovery usually occurs within one to two weeks following transplantation, although on rare occasions it may be delayed for up to three months.
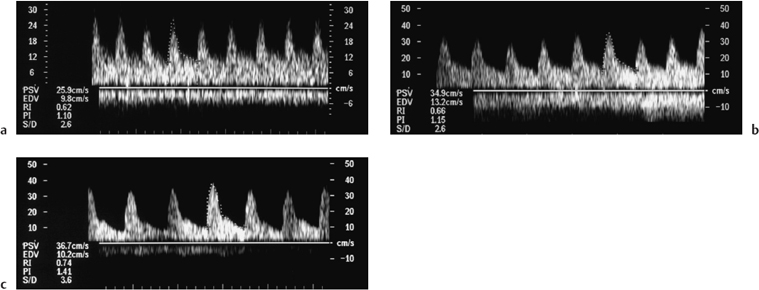
Fig. 4.5a–c Series of spectral Doppler images performed over the course of five days in a patient with delayed function. Normal diastolic flow is demonstrated on all three scans with normal PIs of 1.10, 1.15, and 1.41 respectively. The presumptive diagnosis was acute tubular necrosis (ATN), biopsy was withheld, and the kidney began to function one day after the last scan. There had been no change to the immunosuppres-sive regime in that time
Acute Rejection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree