Fig. 23.1
Plain abdominal film showing kink of the left iliac limb of an aorto-biiliac EVAR
2 Ultrasound
Ultrasound is a noninvasive method that does not use ionizing radiation. It is inexpensive and can be rapidly performed bedside, thus being the method usually chosen for screening programs [23]. Ultrasound can be performed transthoracically for imaging of the ascending aorta or transabdominally for imaging of AAA. Trans-esophagic ultrasound (TEE) allows the imaging of the ascending, parts of the arch, and descending aorta. Due to its higher invasiveness, TEE has been mostly applied intra-operatively during endovascular repair of type B dissections to identify the position of the guidewire in the true lumen and the flow in the true and false lumen.
The use of ultrasound in an emergency situation when suspecting a ruptured AAA, though feasible [24], provides limited information on detailed anatomy. Moreover, it has been reported to slightly underestimate aneurysm diameter when compared to computed tomography [25–27].
The diagnostic sensitivity and specificity of ultrasound to diagnose and follow aneurysms larger than 3 cm exceed 90% [28, 29]. Ultrasound is nevertheless observer dependent and there is a need for standardized protocols and education [30, 31]. These should include a clear definition of the measurement method since this seems to slightly influence the reproducibility of the method [32]. The reproducibility of the method is very high and ultrasound is therefore also useful for control after finding a dilated aorta. The frequency of the exams should increase with increasing aortic diameters [17].
Ultrasound has also been widely used for the follow-up after EVAR of AAA. This may be based on diameter measurement with CT whenever expansion is suspected [33], but some authors also suggest the combined use with duplex to identify endoleaks, which may be further potentiated by the use of contrast enhancement [34]. Ultrasound has a high temporal resolution which has been utilized in analyzing aneurysm pulsatility, especially in the follow-up after EVAR. The clinical value of the pulsatile wall motion is nevertheless limited [35, 36].
The latest developments in ultrasound that may have the potential of giving some advantages in aneurysm imaging include the use of targeted contrast agents against important pathogenic elements such as P-selection. Moreover, 3D ultrasound imaging seems to be possible with rapid acquisition and may allow follow-up of the AAA after treatment. This has the potential of increasing the anatomic detail obtained in terms of special resolution in the preoperative assessment of AAA. The value of the application of this technology remains to be demonstrated.
3 Intravascular Ultrasound
Intravascular ultrasound (IVUS) is an invasive application of ultrasound where a high-frequency probe is mounted on a catheter introduced into the vasculature. It has been widely used for occlusive disease especially in the coronaries where different modalities such has virtual histology possibilities are being developed [37, 38].
Automated vessel analysis has been developed to study the sealing zones in preoperative aortic aneurysm imaging [39]. IVUS has not gained the same popularity for aneurysm imaging mostly due to its invasiveness and the uncertainty in length measurements in patients with tortuous anatomy since the IVUS probe may follow a different path compared to grafts which may condition the length measures. IVUS has been used with some success during EVAR procedures [40], but the advantages seem modest when modern angiographic equipments are used. Some authors have found IVUS to be a good complement during endovascular repair of type B dissections that extend into the abdominal aorta, since it may assist in the assessment of the guidewire position in the true or false lumen [41].
4 Computer Tomography
CT is based on the use of a series of 2D X-ray data obtained around a single axis of rotation that are computed into 3D imaging through digital geometrical processing. The first CT scans provided single axial reconstructions with relatively poor definition. The development of the technique was fast and multidetector-row CT has not only increased the resolution of the technique but also made the exam faster [42]. This allows the performance of rapid CT scans even in the acute setting for the diagnosis of aneurysm rupture while the patients are still hemodynamically stable, which usually is the case when they are admitted to the hospital (Fig. 23.2) [43].
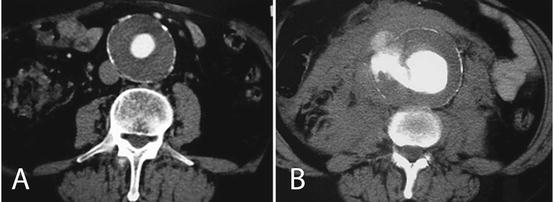

Fig. 23.2
Axial contrast-enhanced CT-scans showing non-ruptured (a) and ruptured (b) abdominal aortic aneurysms
CT of the aorta includes a scan performed with intravenous iodine contrast enhancement. This is obtained by the peripheral venous injection followed by a saline bolus injection. The contrast dose is adjusted according to patient weight. The timing of the scan adjusted by the placement of a region of interest (ROI) in the ascending or aortic arch when the entire aorta is being studied and at the level of the diaphragm if only the abdominal aorta is of interest. Consecutive single scans at the level of the ROI are done and as soon as the Hounsfield unit (HU) threshold is surpassed the scan is started. Patients with renal insufficiency are difficult to exam with intravenous contrast due to the risk of contrast induced nephropathy. This can be dealt with using intra-arterial injection through a transfemoral catheter. thus reducing the contrast dose. Low-dose CT protocols with reduced contrast dose have been used in other areas with good diagnostic result [44], but the applicability in aortic aneurysm imaging is still unclear.
4.1 Preoperative Imaging
Preoperative CT is useful for the assessment of the aneurysm size even in patients where ultrasound imaging is impossible. Diameter measurements can be done without contrast. However, a complete preoperative assessment of the aortic anatomy by the CT should include scans with and without contrast and have thin axial reconstruction (≤1 mm). The scan should be in the arterial phase and extend from the skull base to the lesser femoral trochanter. This will allow mapping of the supraaortic circulation in cases of thoracic aneurysms as well as the access vessels. The assessment of the supraaortic circulation is of importance since it allows evaluation of anatomic variations such as separate origin of the left vertebral artery or aberrant right subclavian artery. Moreover it depicts the completeness of the circle of Willis and thereby assists in the decision on the need for revascularization of the left subclavian artery. CTs done for dissections or aneurysms involving the arch and the ascending aorta are preferably done with EKG-gated technique in order to avoid movement artifacts. Furthermore the EKG-gated CT offers the possibility of triple rule-out diagnostic [45]. Retrospective EKG-gated CT scanning allows for dynamic imaging of the aorta, while maintaining the radiation dose comparable to conventional CT. Dynamic CT can also identify pulsatile changes in the thoracic and abdominal aorta during the heart cycle [46]. The significance of these findings and their implications for stentgraft oversizing during planning as well any consequences for the long-term durability are still uncertain. Other applications of CT imaging are computational post-processing for the refinement of the rupture risk using finite element analysis [47].
4.2 Postoperative Imaging
EVAR relies on the remote insertion of the stent-graft without disrupting the physical integrity of the aneurysm wall. Aneurysm size can, therefore, be used as an indicator of the effectiveness of EVAR in a similar way as it was for the preoperative evaluation of the rupture risk. Aneurysms that become well excluded after EVAR are expected to decrease in size (Fig. 23.3). Consequently, imaging methods have been used for the periodical follow-up after EVAR, evaluating aneurysm size, stent-graft integrity, and/or migration and endoleak status. The use of spiral CT scans for the postoperative follow-up after aortic aneurysm repair was very frequent during the first 15 years after the introduction of EVAR. Currently there has been a trend for changing follow-up after standard EVAR of AAA replacing CTA with ultrasound. However, CT is still the cornerstone for thoracic and more complex EVAR.
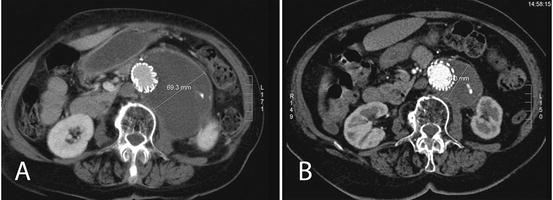

Fig. 23.3
Axial contrast-enhanced CT scans showing aneurysm shrinkage between the 1-year postoperative scan (a) and the 2 year’s one (b)
Follow-up CTA scans are performed with nonionic contrast medium enhancement as described above for the preoperative work-up. In addition, the scan is repeated 60 s later to obtain a delayed scan. The inclusion of a delayed scan to obtain a late arterial phase has the potential of identifying type II endoleaks that could otherwise remain unnoticed [48]. Even during follow-up, low-dose CT protocols can be used to reduce radiation in the repeated exams, without affecting the diagnostic outcome [49].
The increase of the number of detectors up to 320 rows has increased the amount of information and the time required to obtain it. The other significant development in CT scanners in recent years has been the introduction of dual energy CT also called dual source. This consists of two X-ray tubes that simultaneously transmit at different energy levels (usually 80 and 140 kV). This will give different attenuations at the same time and has the potential to reduce the radiation of the exams during EVAR follow-up since it permits the production of virtual non-enhanced images by subtraction of the contrast medium which will allow the identification of endoleaks without the need of a non-enhanced scan [50–54].
4.3 Data Post-processing
As mentioned above, the introduction of multidetector CT scanning increased the definition of the imaging and allowed for 3D imaging which increased the post-processing possibilities. Axial, coronal, and sagittal reconstructions are currently a standard of CT imaging. The transfer of the DICOM raw images to workstations specifically dedicated to vascular imaging post-processing increases the possibilities of the imaging analysis vastly. This is important when planning for EVAR, especially when more advanced prostheses such as fenestrated and branched endografts are needed. It is important to emphasize the need for good quality raw imaging to be able to perform good post-processing. This means high-resolution imaging with thin axial reconstruction of less than 1 mm with good arterial phase enhancement.
Data post-processing allows the reconstruction of multiple multiplanar reconstructions (MPRs) as well as maximum intensity projections (MIPs) with free rotational ability at different angles allowing detailed assessment of different parts of the anatomy such as aortic branch takeoff. These may be useful to plan for the EVAR procedure including the best projections to visualize the different vessels [55]. Another tool of particular importance for EVAR planning is the center line of flow analysis (CLF, Fig. 23.4). This involves the manual or semiautomatic creation of a CLF and thereafter length measurements along that line with evaluation of orthogonal reconstructions along the CLF. Length measurements along CLF are helpful in determining the length of standard grafts since the aorto-iliac segment tends to elongate and become more tortuous upon aneurysm development which makes it difficult to measure lengths in the traditional axial, coronal, or sagittal reconstructions [56]. Moreover, when planning more complex endografts CLF lengths are also helpful in assessing the distance between the different aortic branches that are included in endovascular repair (Fig. 23.4c). The position of the target vessel fenestrations on the endograft also needs to be planned according to their position on the circumference (clock position) and the orthogonal reconstructions can be very useful in very tortuous cases (Fig. 23.4d, e). All CLF measurements are dependent on the accuracy of the graft following the CLF and this is not always the case, especially in cases of high tortuosity. For this reason, one needs to check the CLF path and manually adjust it whenever needed before performing the measurements.
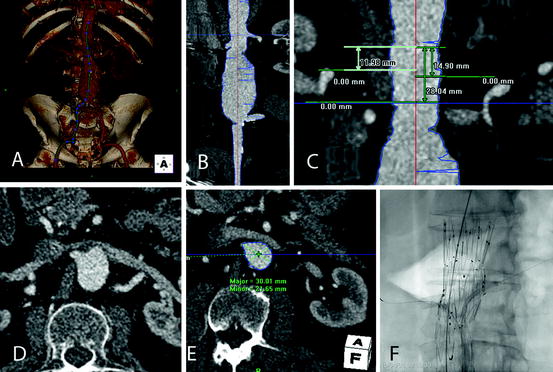

Fig. 23.4
(a) Semiautomated creation of a central line of flow (CLF). (b) Stretch vessel view allowing for length measurements along the CLF. (c) Measurement of the distances along the CLF between the different visceral vessels for the planning of fenestrated stent-graft. (d) Axial slice showing the origin of the right renal artery. The aortic lumen is oval in its appearance due to the postero-anterior angulation of the segment making it difficult to decide the clock position of the vessel origin. (e) Correspondent right renal artery origin in orthogonal reconstruction. (f) Intra-operative image with balloon-expandable stent-grafts deployed in the renal arteries through the respective fenestrations
Post-processing is also very useful in the follow-up after EVAR. Applications such as the creation of multiple MIPs with different window settings and projections will allow assessment of stent-graft integrity and possible dislocation. Fly-through applications that allow virtual angioscopy assist in the assessment of the different branches and possible compression or kinking of stents or stent-grafts [57] CLF is useful even in the assessment of stent-graft migration especially when the endograft is located in a tortuous region such as the distal aortic arch [58]. The use of workstations with the possibility of simultaneous visualization of different exams is very useful during the follow-up after EVAR since it allows the synchronization of images at the same level and in that way evaluation of any changes during follow-up including the evolution of the aneurysm size. This synchronization is also very useful using scans with and without contrast to distinguish between calcifications and endoleaks. Moreover, workstations also provide different methods of measuring the aneurysm size, such as axial diameter, orthogonal diameter, and volume of the aneurysm size. These methods seem to have increasing reliability in the assessment of the evolution of the aneurysm size during follow-up [27, 59, 60], but also require increasing amount of post-processing. A good strategy is therefore to successively take advantage of these different methods in doubtful cases, but using axial diameter measurements in the vast majority. These should be done perpendicular to the longest diameter at the chosen level in order to avoid overestimation caused by tortuosity.
5 Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is another advanced imaging technique. It is possible to use it in a similar way as CT scanning in order to obtain complete imaging of the aorta. It has, nevertheless, not gained the same popularity as CT due to its cost, lower availability, and longer time required for scanning.
MRI is based on the use of magnetic fields created by the imaging equipment that thereafter uses the different relaxation times of the different tissues finally converting them to images. Gadolinium contrast agents can be used to shorten the T1 (longitudinal) relaxation time of and thereby enhance the contrast and shorten the examination time [61]. This is nowadays applied for dynamic MR angiography which is the most common method for the evaluation of the aortic anatomy [62, 63].
The most common presentation of MR angiography images is subtracted MIP. These images are similar to the ones obtained during conventional angiography depicting only the lumen. The raw images should therefore be available and analyzed in order to be able to measure the aneurysm diameter, identify possible dissection, and assess any other changes surrounding the aneurysm such as inflammatory reactions. MR angiography imaging can be post-processed in a way similar to CT in order to obtain an identical morphological evaluation of the aorta and thereby perform EVAR planning [64]. MRI has also been shown to have high diagnostic accuracy in the follow-up after EVER, especially for the identification of type II endoleaks [65–67]. Moreover, the value of the method seems to be further increased by the use of blood-pool contrast agents [68, 69].
MRI used to be the method of choice for patients with reduced renal function. However, reports on the development of nephrogenic systemic fibrosis (NSF) in patients with renal insufficiency who have received Gadolinium-based contrast medium has made the use more restrictive in this category of patients [70]. The risks seem nevertheless to be small and should be weighed against the need for the examination [71]. The preliminary results of unenhanced MRI in EVAR planning seem promising [72] and future technical refinements may make this a valid alternative for patients with renal insufficiency. One final remark should be done on the signal void caused by stainless steel endografts or ferromagnetic markers that make MRI inappropriate or difficult for EVAR follow-up on these cases [73].
Another interesting area of MRI is the use of ultrasmall superparamagnetic iron oxide contrast agents (USPIOs). After injection, these particles are slowly taken up by macrophages, which may reflect the biological activity in the aneurysm wall [74–77]. Dynamic and cine MRI have shown promising results similar to the ones obtained with CT imaging [46, 78–81]. These applications together with the possibility of performing EVAR with MRI may be the upcoming areas of development of the technique [82, 83].
6 Digital Subtraction Angiography
Angiography depicts an outline of the arterial lumen only and has, therefore, low capacity in determining the aneurysm diameter, especially when the aneurysm is partially thrombosed or when the wall is not calcified. For the same reasons, digital subtraction angiography (DSA) is inappropriate for evaluating sealing zones. In the past, angiography was used to complete the diagnosis of concomitant occlusive disease in the access vessels and aortic branches, to define anatomic variations or anomalies before open surgical repair, or to assist in length measurement before EVAR. However, this has been replaced by the use of volume rendering software for CT and MR [84, 85] which are noninvasive alternatives and can provide accurate measurements. Angiography is therefore currently used primarily to guide interventions. Hybrid rooms are the ideal environment for the performance of EVAR since it provides an operating room associated with high-quality angiographic imaging. However, current C-arms are feasible alternatives [86].
DSA is usually performed with iodine intra-arterial contrast agents that provide high-quality images but have the drawback of nephrotoxicity. Current angiographic equipments allow adjustments to retain the imaging quality while decreasing the amount and concentration of contrast used [87]. Moreover, carbon dioxide is a good alternative for abdominal EVAR [88, 89], but may be difficult to use for the imaging of dorsally oriented arterial takeoff due to the tendency of the gas to float in the blood. On the contrary it is excellent for arteries with ventral origins, but may should not be used above the diaphragm (Fig. 23.5).
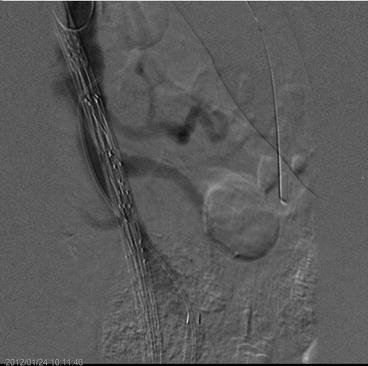

Fig. 23.5
Digital subtraction angiography during the placement of a triple fenestrated stent-graft. Carbon dioxide is used as contrast agent showing the superior mesenteric artery origin
7 Rotation Angiography, Cone Beam CT, and Fusion
Modern angiographic suits with flat digital panels have given the possibility of doing runs to obtain high-definition volume-rendering data. One of the possibilities is to obtain digitally subtracted rotational angiographies that may even be used as overlay reference into the fluoroscopy. This has limited application in aneurysm imaging since it conveys only an outline of the contrast-filled. However, it can be useful for intra-procedural orientation and characterization as well as during reinterventions for intraluminal endoleak embolization.
The other alternative is to use the flat panel as a CT detector to perform a cone beam CT (CB-CT). This has a high spatial resolution but the volume is limited to the length of the detector and centered in the iso-center. Good quality imaging can be obtained with low dose of iodine contrast. Preliminary results from the application of this technique seem promising compared to the preoperative anatomic evaluation [90–92] and in the assessment of the intra-operative final result (Fig. 23.6) [93]. CB-CT has limited use in the routine follow-up due to its invasive nature with an intra-arterial injection. However there is high potential in the assessment of the reintervention result especially when the location of the failure is difficult to visualize on standard DSA. In the current setup, CB-CT will not be able to totally replace the standard preoperative CTA or MRA since these are able to depict the entire aorta and access vessels while CB-CT is limited to the cone area as mentioned above.
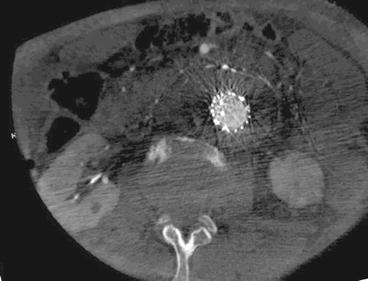

Fig. 23.6
Axial reconstruction of contrast-enhanced cone beam CT as intra-operative control after EVAR deployment
Another technical advancement that seems to have great potential is the ability to obtain fusion of the preoperative CTA data with the fluoroscopy. This is achieved by matching the bony references of the preoperative CTA with a non-contrast-enhanced CB-CT. In this way the software will be able to use the preoperative CTA as an overlay reference in the fluoroscopy screen. There were some theoretical concerns on the potential deviation of the anatomy by the insertion of stiff large bore introducer in the aorta which could be misleading. The first results of the application of this technique in branched and fenestrated EVAR are nevertheless very promising [94]. Other applications are being developed with similar results [95].
8 Integrated Positron Emission Tomography and Computed Tomography
Aortic imaging with positron emission tomography (PET) uses fluorine 18-fluorodesoxyglucose (FDG) that is taken up by metabolically active cells such as activated leukocytes in the arterial wall [96, 97]. FDG disintegrates resulting in positron and gamma ray emission which are then detected by the cameras. Multidetector CT scanners can currently be used in integrated equipments with PET. This provides high-quality fusion imaging with the detailed morphological imaging of the CT and the functional information of the PET. The results of positron emission tomography and computed tomography (PET-CT) in pre- and postoperative imaging of aortic aneurysms have been contradictory and further studies are needed to establish the value of the technique [98–105].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree