Acknowledgments
I acknowledge Brad Hoehne, Radiology Digital Imaging Specialist (Nationwide Children’s Hospital), for preparation of figures.
Back pain is common in children and tends to increase with age, especially in the early teen years. Although back pain can be the presenting symptom of serious pathology, such as infection or neoplasm, recent studies have shown lower rates of identifiable disease in children with back pain. A nonpathological cause, such as mechanical low back pain, is frequently seen in children and responds to conservative treatment without requiring diagnostic imaging. A detailed clinical history and a thorough physical examination are critical to avoid unnecessary workups, and can identify patients with clinical red flags who will benefit from further radiographic or laboratory studies. Familiarity with common disorders that manifest with back pain in children, and the use of a systematic approach to choose the indicated imaging modality will lead to a correct diagnosis. This chapter will review the epidemiology, clinical evaluation, imaging workup, and radiological findings of common and a few rare disorders that cause back pain in children.
Epidemiology
There is wide variability in the reported prevalence of back pain in children, ranging from 12% to 50%. Difficulty in determining the prevalence of back pain in children includes several factors, such as differences among studies in defining “back pain” (some refer to back pain in general, while others refer exclusively to low back pain), variability in the time period assessed, sample size, and a small number of prospective studies on the epidemiology of back pain in children. Prevalence increases with age, and specifically low back pain shows a dramatic increase in the early teen years. Lifetime prevalence rate has been shown to increase from 12% at 11 years of age to 50% at 15 years of age. A 40.2% lifetime prevalence of back pain in children between 10 and 16 years of age has also been reported. Suggested risk factors include females, poor fitness in sedentary adolescents, and family history. The adolescent athlete can be considered a different subgroup in which low back pain more often results from acute injury or overuse injury.
Back pain in children is most often a symptom of a benign process, but it can also be the manifestation of a more serious pathology, including infectious/inflammatory, neoplastic, congenital, and traumatic etiologies ( Table 30.1 ). Mechanical low back pain is common in children and refers to pain without an identifiable etiology on physical examination, radiographs, or advanced imaging evaluation. This condition responds to conservative treatment and usually does not require imaging evaluation.
|
Clinical Evaluation
A detailed clinical history and a thorough physical examination are essential in identifying the source of back pain in children and determining whether further radiographic or laboratory studies are necessary. Children who have no significant physical findings, short duration of pain, and a history of minor injury can be treated conservatively without further work up. Pain should be characterized in terms of site, duration, frequency, mechanism of onset, time of onset (e.g., nighttime), acute or chronic nature, radiating pain, and association with recent-onset scoliosis. Pain associated with constitutional symptoms, such as fever, malaise, and night sweats, may suggest underlying infection or malignancy. Pain that improves with aspirin or nonsteroidal antiinflammatory drugs (NSAIDs) can be consistent with an osteoid osteoma. Clinical red flags, including constant pain, night pain, radicular pain, pain lasting longer than 4 weeks, and abnormal neurological examination should prompt further imaging evaluation. Laboratory tests are indicated in patients with suspicion for infection or systemic illness and must include inflammatory markers and a complete routine blood panel. If these tests are positive, imaging evaluation is indicated as well.
Imaging Evaluation
The American College of Radiology recently published an Appropriateness Criteria guideline for imaging evaluation of back pain in children ( ). These guidelines and recommendations discuss the indications for imaging modalities according to different clinical variants. These variants include: (1) initial imaging evaluation in the child without clinical red flags; (2) initial imaging evaluation in the child with one or more clinical red flags: (3) child with negative radiographs and one or more clinical red flags; (4) child with positive radiographs and one or more clinical red flags; (5) child with chronic back pain associated with overuse (mechanical back pain); and (6) child with back pain associated with suspected inflammation, infection, or malignancy.
Radiographs
Radiographs of the entire spine are a good initial diagnostic tool for the evaluation of back pain in children and include anteroposterior and lateral views. Oblique views should not be obtained routinely in children, because these do not increase sensitivity while adding considerable radiation exposure. Radiographs are not necessary in children with short duration of symptoms that respond to conservative therapy. In 24% of cases, radiographic findings have been shown to lead to the diagnosis. Radiographs may detect bony lesions, such as vertebra plana, fractures, Scheuermann disease, spondylolysis, scoliosis, and expansile and destructive lesions like aneurysmal bone cysts (ABCs).
Advanced Imaging: Computed Tomography, Magnetic Resonance Imaging, and Nuclear Medicine
After radiographs, the best imaging technique for the evaluation of back pain in children is variable. The choice of computed tomography (CT), magnetic resonance imaging (MRI), or bone scintigraphy depends on the clinical presentation, suspected underlying pathology, and the child’s age. Another important consideration when imaging children is the amount of radiation exposure with each technique. Ionizing radiation places children at risk for development of neoplasms and therefore should be kept to a minimum. Pediatric patients are more susceptible to the mutagenic effects of radiation, and their lifetime risk is greater because they have more years to live and therefore accumulate more radiation exposure throughout their life. The ALARA (As Low As Reasonably Achievable) principles must always be applied, and whenever possible, imaging modalities that do not use ionizing radiation, such as MRI, should be considered.
Computed Tomography
The use of CT in children should be limited because of its ionizing radiation, and when indicated, CT must be restricted to the smallest field of view possible. CT is considered the imaging technique of choice for characterizing fractures and bony lesions. Two-dimensional reformatted images and three-dimensional reconstruction models are useful in the preoperative evaluation of spinal trauma and scoliosis and for presurgical orthopedic planning.
Magnetic Resonance Imaging
MRI is the imaging technique of choice in diagnosing intraspinal or paraspinal pathology and is indicated in children with back pain and one or more clinical red flags. In patients with abnormal neurological findings, initial evaluation with MRI of the entire spine may be indicated. When there is suspicion of infection, inflammation, or neoplasm, precontrast and postcontrast MR sequences should be performed to assess patterns of enhancement. Generally, noncontrast MRI is done in the child with low back pain with suspected spondylolysis or disk pathology. The decision of using MRI must be weighed carefully against its relatively high cost and the need for sedation in younger children.
Technetium-99m Bone Scan Whole Body With Single-Photon Emission Computed Tomography Spine
Technetium-99m ( 99m Tc) methylene diphosphonate bone scintigraphy with SPECT (single-photon emission computed tomography) is another tool in the imaging workup of back pain in children. It has high sensitivity in detecting spondylolysis and is superior to MRI in detecting active spondylolysis. Bone scans are particularly useful in identifying multifocal disease as well. However, bone scans expose patients to significantly more radiation than radiographs and CT. In particular, the urinary bladder is subjected to a large amount of radiation because the radioactive tracer is excreted through the urine and temporarily stored in the bladder before voiding. Coregistered CT is becoming a standard component of modern SPECT. Again, increased radiation exposure with this technique is a limiting factor for its use. Advantages of SPECT/CT over SPECT alone include improved anatomic localization of sites of abnormal uptake, identification of causes of abnormal uptake, and demonstration of bony abnormalities that do not show abnormal radiotracer uptake. Abnormalities of the posterior elements, including lumbar interspinous bursitis, spinous process avulsion, and facet hypertrophy can be better assessed with SPECT/CT.
Spondylolysis and Spondylolisthesis
Spondylolysis refers to a defect or fracture of the pars interarticularis, which is the weakest portion of the neural arch. The pars interarticularis is the bony segment formed by the junction of the vertebral pedicle, the superior and inferior articular processes, and the lamina. Although etiology remains unclear, risk factors include developmental defects and repetitive trauma from excessive hyperextension. A prevalence rate of 4.4% has been reported in children and 6% in the general population. The incidence of spondylolysis increases with the practice of sports such as gymnastics, soccer, volleyball, diving, and ballet. Bilateral involvement is more frequent than unilateral, and the most commonly affected vertebra is L5, followed by L4 and L3. Multilevel involvement can occur, and atypical involvement at a higher level, such as L2, tends to be more painful.
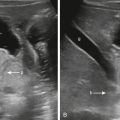
There is no general consensus for the imaging workup to evaluate suspected spondylolysis. However, evaluation typically begins with plain radiographs and can be further assessed with any of the following imaging modalities: MRI, CT, 99m Tc bone scan whole body with SPECT spine, or SPECT/CT. Radiographs are considered a poor screening test with low sensitivity for spondylolysis in children; only two-view (anteroposterior and lateral) radiographs are recommended. It has been demonstrated that oblique views do not increase sensitivity and specificity, but rather add radiation exposure. A pars fracture can be seen as an oblique lucency at the base of the laminae on the lateral view ( Fig. 30.1 ). Radiographs are useful for the evaluation of spondylolisthesis , defined as anterior slippage of a given vertebra over the one below it, in the presence of bilateral pars fractures. The degree of anterior vertebral displacement is graded as follows: grade 1, less than 25% displacement; grade 2, 25% to 50% displacement; grade 3, 50% to 75% displacement; and grade 4, 75% to 100% displacement. Grade 5 (spondyloptosis) refers to complete displacement of the vertebral body anteriorly ( Fig. 30.1A ).

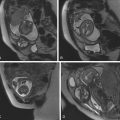
MRI is currently a preferred imaging modality to diagnose spondylolysis due to the lack of radiation and its ability to assess soft tissue and bony structures. The following spectrum of abnormalities of the pars interarticularis can be demonstrated with MRI: focal marrow edema without a visible fracture consistent with stress reaction, incomplete or complete fractures with marrow edema (in the acute/subacute phase), or chronic (nonunion) fractures with no marrow edema. Paraspinal soft tissue edema can be seen occasionally in the acute setting. Incomplete fractures extend from inferior to superior. Marrow edema will show hypointense signal intensity on T1-weighted images (T1WIs), hyperintense signal on T2-weighted images (T2WIs) with fat saturation, or STIR (short-tau inversion recovery) pulse sequence. Sagittal multiple-echo recombined gradient-echo images are also recommended because they provide greater sensitivity for visualization of pars fractures ( Fig. 30.1B ). On axial images, pars fractures have a transverse orientation and should not be mistaken with the adjacent facet joints, which have an oblique orientation. The facet joints are seen one or two slices above and below the pars fractures ( Fig. 30.2C,D ).

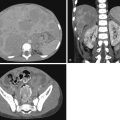
Healing response of incomplete fractures is not well seen with MRI or CT; neither modality can assess whether an incomplete fracture is in an evolutionary or reparative phase. CT scans can best characterize the fracture and can show associated findings, such as bony fragmentation or surrounding sclerosis ( Fig. 30.3 ).

SPECT bone scans are sensitive for detecting spondylolysis and reveal areas of bone turnover; findings are generally positive for a prolonged period (~6 weeks). Spondylolysis presents as increased radiotracer uptake in the posterior elements (pars interarticularis, lamina, or pedicle) ( Fig. 30.4 ) ( ). These findings may be consistent with stress reaction, stress fracture, or a symptomatic spondylolytic defect. Coregistered CT is now a standard component of modern SPECT systems. The advantages of SPECT/CT were mentioned earlier in this review.

The differential diagnosis of spondylolysis includes fractures of the vertebral pedicles, which are significantly less common, but can also show bilateral uptake in the posterior elements with SPECT. Fractures of the pedicle are most often unilateral but can be seen in association with contralateral spondylolysis. Bilateral pedicle fractures tend to occur in skeletally mature patients and are difficult to differentiate from pars fractures with SPECT. Coregistered CT can make this distinction as well as MRI ( Fig. 30.5 ).

Nonsurgical treatment of spondylolysis includes restriction of activity that exacerbated symptoms, activity modification limited to specific forms of exercise (e.g., stationary biking and modified swimming), bracing, NSAIDs, and physical therapy.
Disk Calcification
Calcification of the intervertebral disk is a rare disorder in children of unknown etiology. Proposed causes include inflammation or infection (i.e., diskitis, osteomyelitis), trauma, or metabolic disorders (i.e., hyperparathyroidism). It also has been described in association with syndromes such as Morquio syndrome, I cell disease, and Patau syndrome. Disk calcification occurs more often in the cervical spine, followed by the thoracic and lumbar spine, and has a slight predilection for boys. This entity usually occurs during the first two decades of life and is self-limiting, with most symptoms resolving within 6 months. Disk calcification can completely resolve with time.
Disk calcifications can be seen incidentally or can present with symptoms such as pain, stiffness, decreased range of motion, muscle spasm, tenderness, and torticollis. Fever and increased erythrocyte sedimentation rate (ESR) may also be present. Symptoms tend to be brief and rarely last for more than several weeks. Associated disk herniation may occur as well.
On radiographs and CT, disk calcification is seen as a round, oval, flattened, or fragmented calcification in the nucleus pulposus. On MRI the calcified disk will appear dark on T2WI and bright on T1WI. Associated calcification of the posterior ligament can occur. MRI can best evaluate an associated disk herniation ( Fig. 30.6 ).

Treatment is conservative; however, severe myelopathy or radiculopathy will require surgical intervention.
Scheuermann Disease/Kyphosis
Scheuermann disease is an osteochondrosis of the spine affecting the vertebral epiphyseal growth plates. This condition is the most common cause of progressive thoracic or thoracolumbar angular hyperkyphosis in adolescents and is associated with back pain. Unlike postural kyphosis, this is a rigid deformity that does not correct when the patient is asked to stand straight. The radiological diagnosis consists of the following findings: anterior wedging of three or more contiguous vertebrae of 5 degrees or greater, resulting in a thoracic kyphosis greater than 35 degrees. Other associated findings include irregular end plates with Schmorl nodes, disk-height loss, and associated apophyseal ring fractures ( Fig. 30.7 ).

Prevalence rates range from 0.4% to 10% depending on diagnostic inclusion criteria. Conflicting reports show no consistent sex predilection in this disorder that occurs during the period of rapid bone growth between 12 and 15 years of age. Although pathogenesis remains unclear, genetic and mechanical factors likely are involved.
Clinical presentation consists of pain localized to the midscapular region at the level of the kyphotic deformity that usually intensifies gradually after activity, or after prolonged periods of sitting or standing, and improves with rest. The apex of the kyphotic deformity is more commonly localized to the T7-T9 vertebrae or at the thoracolumbar junction. Atypical Scheuermann kyphosis (lumbar type) is less common and occurs at the thoracolumbar junction with the apex between T10 and T12.
Initial imaging evaluation consists of plain radiographs. CT may be indicated to further evaluate disease or for preoperative planning. MR is useful in evaluating associated apophyseal ring fractures.
Treatment is mainly conservative, particularly for patients with remaining spinal growth and for those who respond well to physical therapy and bracing. Surgical treatment is reserved for patients with severe curvatures (>75–80 degrees) or skeletally mature patients with rigid curvatures.
Sacral Meningocele
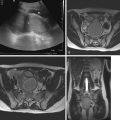
An intrasacral meningocele consists of a cyst of fibrous tissue usually lined by arachnoid, located within an expanded sacral spinal canal that is attached to the caudal termination of the dural sac by a pedicle and allows flow of cerebrospinal fluid (CSF) from the tip of the subarachnoid space into the meningocele. Intrasacral meningoceles do not contain nerve roots, unlike perineural (Tarlov) cysts. Other terms used to refer to this entity include occult intrasacral meningocele, intrasacral cyst, intrasacral extradural arachnoid cyst, intraspinal meningocele, or giant sacral meningeal diverticula. Although these cysts can be discovered incidentally, the most common symptom is chronic, intermittent low back pain. Other symptoms such as urinary incontinence and constipation result from bowel and bladder dysfunction caused by local nerve root compression.
Of the various theories of pathogenesis proposed, the most accepted one is that it results from a primary mesenchymal defect involving the dura. Despite being considered a congenital abnormality, intrasacral meningoceles can expand over time because of hydrostatic pressure. There is an association with other intraspinal abnormalities, such as sacral vertebral anomalies, diastematomyelia, or tethered spinal cord.
MRI is the imaging modality of choice and provides fine anatomic detail for presurgical planning. Findings consist of an extradural, thin-walled cyst with smooth contours located within the sacral spinal canal adjacent to the distal thecal sac, without neural elements in the cyst and following CSF signal intensity on all sequences. Conventional MRI is usually sufficient for diagnosis and will demonstrate a hyperintense structure on T2WI and a hypointense structure on T1WI ( Fig. 30.8 ). However, identifying the site of connection between the cyst and the thecal sac (isthmus or pedicle) can be difficult. Thin-section T2-weighted (steady-state acquisition) imaging can provide additional anatomic detail and can occasionally depict the connection between the cyst and the dura. Usually if the apex of the lesion is midline, it is more likely to be an intrasacral meningocele rather than a perineural cyst that is located eccentrically within an enlarged neural foramen, with dilatation of the nerve root meningeal sleeve. These cysts do not enhance with contrast material. Diffusion-weighted imaging can help differentiate an intrasacral meningocele from an epidermoid/dermoid cyst.