Fig. 9.1
Reduced cerebral cortical oxygen metabolism without morphological changes. A patient with occlusion of the left internal carotid artery showed decreased cerebral blood flow (CBF) and increased oxygen extraction fraction (OEF, misery perfusion) in the hemisphere with occlusion. Surgical reperfusion with bypass improved misery perfusion. However, we should note that cerebral metabolic rate of oxygen, which was decreased in the normal-appearing cerebral cortex before surgery, was unchanged after surgical reperfusion. Some irreversible damage may occur in the cerebral cortex
Although correct evaluations of hemodynamic status are essential to determine patient prognosis, therapeutic strategies to prevent recurrent strokes, which should differ between patients with and without hemodynamic compromise, are not clearly established. We should continue to make efforts to seek strategies for selecting treatments based on hemodynamic measurements.
9.1.2 Selective Neuronal Damage/Loss or Incomplete Infarction
In atherosclerotic ICA or MCA occlusive disease, chronic hemodynamic compromise increases the risk for cerebral ischemic damage, irrespective of the development of symptoms. Hemodynamic cerebral ischemia due to ICA or MCA occlusive disease may cause not only cerebral infarction but also minor tissue damage in the cerebral cortex that is not detectable as infarction on CT or magnetic resonance imaging (MRI). Neuropathological studies have demonstrated cortical granular atrophy or watershed territory microemboli in the cerebral cortex distal to large cerebral arterial occlusion (Graham 1992). Observations in both humans and animals also suggest that selective cortical neuronal loss results from acute occlusion of the large cerebral arteries accompanied by ischemia of moderate severity (Garcia et al. 1996; Lassen et al. 1983). However, it is unclear whether selective neuronal ischemic change develops in patients with atherosclerotic occlusion of the large cerebral arteries, because a direct demonstration of the cortical neuronal loss in the living brain is not possible.
To address the issue of neuronal loss without infarction in ICA or MCA occlusive diseases, indirect markers for neuronal damage including brain atrophy and decreased metabolism have been investigated. A decrease in glucose or oxygen metabolism in the normal-appearing cerebral cortex is one result of ischemic damage to the tissue, but it can also be caused by a decrease in neural input from distant regions with fiber connections (Feeney and Baron 1986). Atrophy of the corpus callosum may be an indirect but sensitive indicator of ischemic cortical neuronal loss. The largest fraction of neurons projecting into the corpus callosum is the large pyramidal cells in layer 3, which is one of the groups of cortical neurons most vulnerable to ischemia (Graham 1992). Therefore, callosal atrophy may result from cortical ischemic loss. Reduced cerebral cortical oxygen metabolism in the normal-appearing cerebral cortex, which surgical reperfusion cannot improve, has been demonstrated in occlusive diseases of ICA or MCA by using PET, suggesting that selective neuronal damage may occur (Powers et al. 1984; Yamauchi et al. 1990). Our studies showed that a decrease in bilateral cerebral cortical oxygen metabolism occurs in association with callosal atrophy in patients with ICA occlusive disease, suggesting that ischemic neuronal loss may contribute to the reduced cortical oxygen metabolism (Yamauchi et al. 1993, 1995, 1996a, 2000b). However, to address this issue further, a more direct marker for neuronal damage was needed.
9.2 Imaging of Central-Type Benzodiazepine Receptors
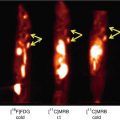
Development of imaging techniques makes it possible to visualize the distribution of central-type benzodiazepine receptors (cBZR) in humans by using 11C-labeled flumazenil (FMZ) for PET or 123I-labeled iomazenil (IMZ) for single-photon emission computed tomography (SPECT) (Beer et al. 1990; Persson et al. 1985; Samson et al. 1985). Central BZR is coupled with γ-aminobutyric acid receptors (GABAA/BZ receptor complex), and cBZR exists in the membrane of neuron (Olsen 1981). Central BZR is expressed by most cortical neurons. Binding of FMZ is high in the cerebral cortex and is low in the cerebellum, thalamus, and basal ganglia, while it is lacking in the white matter (Fig. 9.2). As shown in the case of crossed cerebellar diaschisis, a simple decrease of neural input may not decrease cBZR (Dong et al. 1997; Hatazawa et al. 1995a). Thus, imaging of the cBZR may detect cortical neuronal damage, which could not be evaluated directly by other neuroimaging modalities (Garcia et al. 1996; Hatazawa and Shimosegawa 1998; Sette et al. 1993). Cortical neuronal damage should lead to a decrease in cBZR.
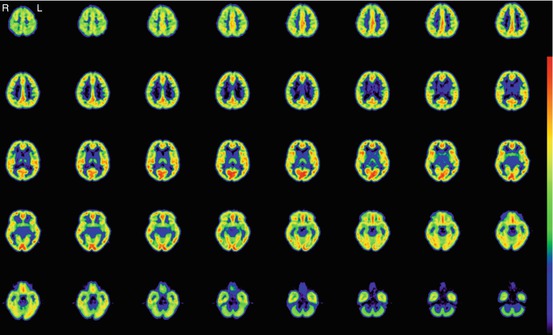

Fig. 9.2
BZR imaging with 11C-Flumazenil PET. Images of a male normal volunteer of 49 years old show high binding in the cerebral cortex
Histopathological correlations between decreased cBZR and selective neuronal loss were confirmed recently (Ejaz et al. 2013). A rat ischemia-reperfusion model with 45-min occlusion of the distal MCA was examined with FMZ PET and immunohistochemistry 28 days after ischemia. The study demonstrated that a decrease in FMZ binding in the normal-appearing cerebral cortex on T2-weighted MRI was associated with selective neuronal loss on stains with antibody for neurons. Imaging of cBZR by using 11C-FMZ and PET can detect selective neuronal damage or loss.
9.3 Pathophysiology of Selective Neuronal Damage Demonstrated as Decreased BZR
9.3.1 Selective Neuronal Damage and Chronic Hemodynamic Cerebral Ischemia
Several small studies in patients with atherosclerotic ICA or MCA disease in the chronic stage demonstrated a reduction in cBZR in the normal-appearing cortical areas on CT or MRI, suggesting the presence of selective neuronal damage (Dong et al. 1997; Kuroda et al. 2004, 2006; Moriwaki et al. 1998; Sasaki et al. 1997; Yamauchi et al. 2000a). The IMZ uptake on SPECT was significantly but weakly correlated with the cerebral metabolic rate of oxygen, while no significant correlation was found of the IMZ uptake with the blood flow, the cerebrovascular reactivity, or the cerebral metabolic rate of glucose, which suggested that the reduction of IMZ uptake may be due to selective neuronal loss that may not be revealed by other imaging tracers (Dong et al. 1997; Moriwaki et al. 1998; Sasaki et al. 1997). These findings were also shown by using FMZ and PET (Kuroda et al. 2004, 2006). A decrease in cortical BZR binding was correlated with corpus callosum atrophy, which supported that decreased cortical BZR binding may reflect cortical neuronal damage (Yamauchi et al. 2000a).
However, the incidence, extent, and severity of selective neuronal damage demonstrated as decreased cBZR are unclear. Furthermore, the factors responsible for the decreased cBZR are not well understood. Specifically, although a chronic hemodynamic mechanism may be responsible for the decreased cBZR, the relationship between the decreased BZR and chronic hemodynamic compromise is unknown. The clarification of this relationship is important because vascular reconstruction surgery can improve chronic hemodynamic compromise, which may prevent the development of selective neuronal damage as well as infarction.
9.3.2 Selective Neuronal Damage and Low-Flow Infarction
Borderzone infarction has been demonstrated to be associated with ICA occlusive disease and hemodynamic compromise (Momjian-Mayoy and Baron 2005; Yamauchi et al. 1990, 1991). Hemodynamic ischemia due to ICA occlusive disease may cause not only borderzone infarction but also selective neuronal damage beyond the regions of infarcts that may be detected by a decrease in cBZR in the normal-appearing cerebral cortex. To determine whether selective neuronal damage is associated with borderzone infarction in ICA occlusive disease, we measured cBZR using PET and 11C-FMZ in 62 nondisabled patients with ICA steno-occlusive lesions in the chronic stage (Yamauchi et al. 2005). FMZ-binding potential (BP) was calculated using the dynamic data and Logan graphical analysis with the reference tissue, with the pons as reference region (Logan et al. 1996; Okazawa et al. 2004). The infarcts on MRI, which were categorized as territorial, borderzone (external or internal), striatocapsular, lacunar, and other white matter infarcts, were correlated with mean cerebral/cerebellar cortical BP ratio in the hemisphere with ICA occlusive disease. Patients with borderzone infarction (n = 18) had significantly decreased FMZ-BP ratio in the hemisphere with ICA disease, compared with patients without borderzone infarction (n = 44) and normal control subjects (n = 10). Both external and internal borderzone infarctions were associated with the decreased FMZ-BP ratio. Multivariate analysis showed that external borderzone infarction was an independent predictor of the decreased FMZ-BP ratio. In ICA occlusive disease, selective neuronal damage demonstrated as decreased cBZR is associated with borderzone infarction. Microemboli can cause borderzone infarction, particularly in cases of ICA stenosis (Moustafa et al. 2010). However, it is less likely that borderzone infarction due to microemboli causes extensive decreases of BZR beyond the regions of the infarct, because ischemia due to microemboli is restricted to the borderzone region. Therefore, hemodynamic ischemia leading to borderzone infarction may cause selective neuronal damage beyond the regions of infarcts in the chronic stage.
Decreased cBZR was closely associated with decreased oxygen metabolism. However, the correlation of decreased cBZR with increased OEF (hemodynamic compromise) was relatively weak. Selective neuronal damage caused by hemodynamic ischemia in turn decreases the degree of hemodynamic compromise due to ICA occlusive disease through causing reduced metabolic demand of the tissue (Kuroda et al. 2004; Yamauchi et al. 2004), which may lead to normalization of OEF after the development of selective neuronal damage. The association of decreases in cBZR with borderzone infarctions suggests that severe hemodynamic compromise, which was present in the acute stage of infarction, may have disappeared according to the decrease of the metabolic needs of the tissue between the time of infarction and the PET examination. Careful medical managements in the acute stage for patients with borderzone infarction should prevent selective neuronal damage, even when infarct size is small and symptom is mild. Then, the preserved metabolic needs of the cortical tissue in the chronic stage may permit the persistence of the hemodynamic compromise that can be improved by vascular reconstruction surgery. This strategy results in preservation of cortical metabolism that may lead to good functional outcomes. Recognition and prevention of selective neuronal damage are important for the management of patients with ICA occlusive disease.
The association between borderzone infarction (internal borderzone infarction) and a decrease in cBZR in the overlying cerebral cortex was also found in 62 nondisabled patients with atherosclerotic occlusive disease of the MCA and no cortical infarction (Yamauchi et al. 2009).
9.3.3 Selective Neuronal Damage and Misery Perfusion
9.3.3.1 A Cross-Sectional Study
The notion that normalization of OEF may occur after the development of selective neuronal damage suggests that it may be not easy to demonstrate an association of decreased cBZR with chronic hemodynamic compromise (increased OEF) in the chronic stage. To overcome this issue, we studied 105 nondisabled patients with atherosclerotic ICA or MCA occlusive disease and no cortical infarction on routine MRI images (T1-weighted, T2-weighted, or fluid-attenuated inversion recovery images) and measured cBZR and OEF using PET. We investigated the association of selective neuronal damage demonstrated as a decrease in cBZR in the normal-appearing cerebral cortex with increased OEF (misery perfusion) (Yamauchi et al. 2007).
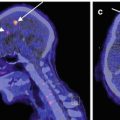
A three-dimensional stereotactic surface projection (3D-SSP) technique was used to analyze FMZ-BP (Minoshima et al. 1995). After anatomical standardization, a method of data extraction is performed in which the cortical activity is projected onto the brain surface. The algorithm searches the highest pixel value in a direction inward along the vector to a six-pixel depth into the cortex on an individual’s anatomically standardized PET image set and assigns the maximum value to the surface pixel. The surface projection technique minimizes the effect of cortical atrophy. Then, a comparison of the resultant cortical projections between a patient and controls is performed. To quantify a decrease of FMZ-BP, pixel-by-pixel z-scores were used; z-scores (([mean normalized pixel value of controls] − [normalized pixel value of each patient])/[SD of controls]) were calculated for each surface pixel (z-score map) (Fig. 9.3). A positive z-score represents a reduced FMZ-BP in patients relative to controls. To assess the degree of abnormal FMZ-BP reduction in each patient quantitatively, the BZR index [(the extent of the pixels with z-score more than 2 compared with controls) × (average z-score in those pixels)] in the cerebral cortex of the MCA distribution with arterial disease was calculated by the stereotactic extraction estimation (SEE) method (Mizumura et al. 2003).
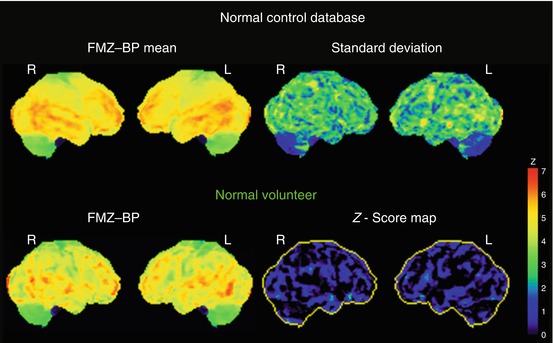

Fig. 9.3
Three-dimensional stereotactic surface projection (3D-SSP) analysis. Upper row. 3D-SSP images showing the value of mean and standard deviation for normal control database (n = 10, mean age 57 years). Lower row. 3D-SSP images and z-score maps of a male normal volunteer of 49 years old shown in Fig. 9.2
All patients had pixels with abnormally decreased cBZR in the cerebral cortex of the MCA distribution, with the extent varying from 0.04 to 60.91 % (Fig. 9.4). The BZR index was significantly correlated with the mean hemispheric value of OEF or CBF in the ipsilateral hemisphere. Multivariate analysis showed that the BZR index was positively correlated with the value of OEF and the history of stroke, which suggested that misery perfusion might cause selective neuronal damage. On the other hand, the BZR index was negatively correlated with the presence of statin treatment. Several studies have shown that statins have beneficial effects on the cerebral circulation and brain parenchyma during ischemic stroke and reperfusion (Vaughan and Delanty 1999). The antioxidative properties of statins might be involved in their beneficial effects against neuronal damage in cerebral ischemia (Nagotani et al. 2005). Statins might also reduce microemboli by plaque stabilization in patients with ICA or MCA stenosis. Therefore, statins may reduce the occurrence of selective neuronal damage due to ischemia in atherosclerotic ICA or MCA occlusive disease.
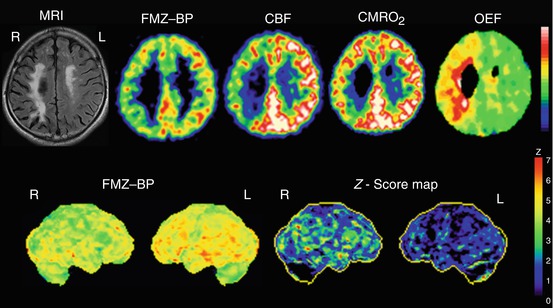

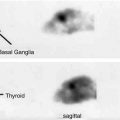
Fig. 9.4
Selective cortical neuronal damage and misery perfusion. Upper row. Examples of PET images showing a decrease of flumazenil-binding potential (FMZ-BP), cerebral blood flow (CBF), and cerebral metabolic rate of oxygen (CMRO 2 ) with increased oxygen extraction fraction (OEF) in a patient with right (R) internal carotid artery occlusion who showed internal borderzone infarction on the corresponding MRI images. This patient presented with left hemiparesis and spatial neglect. Lower row. 3D-SSP images and z-score maps showing a decrease of FMZ-BP in the right hemisphere. The extent of abnormally decreased benzodiazepine receptors (BZR) was 47.6 % in the right middle cerebral artery distribution; the mean z-score in those pixels was 2.92; and the BZR index was 139.2
9.3.3.2 A Follow-up Study
To demonstrate a causal relationship between misery perfusion and selective neuronal damage more directly, it is essential to show a correlation between an increase of OEF and progression of selective neuronal damage using a longitudinal study. Therefore, follow-up PET examinations in 17 selected patients without ischemic episode were performed to investigate the relationship between changes in cerebral hemodynamics and the decrease in cBZR (Yamauchi et al. 2007). The interval between the first and follow-up PET studies ranged from 2.5 to 39 (mean 18 ± 9) months. The total change of the BZR index or the OEF value in the cerebral cortex of the MCA distribution with arterial disease was calculated by subtracting the value obtained at the second scanning from that obtained at the first scanning. The relation between the change of the BZR index and that of the OEF value was analyzed. T2-weighted MRI disclosed extension of the confluent white matter lesions preexisted at the first evaluation in one stroke patient. The other 16 patients showed no apparent changes on the follow-up MRI images. The BZR index increased significantly between the first and second examinations from 25.61 ± 32.79 to 50.75 ± 76.11. The degree of increase in the BZR index was positively correlated with that in the OEF value. Follow-up data indicate that hemodynamic deterioration causes selective neuronal damage demonstrated as decreased BZR without overt episode of stroke. This study confirmed the relationship between selective neuronal damage and chronic hemodynamic compromise for the first time. In patients with chronic hemodynamic compromise, the perfusion may fall below the penumbra threshold for a matter of minutes, causing selective neuronal damage.
9.4 Silent Cortical Neuronal Damage in Asymptomatic Patients
The finding that selective neuronal damage manifested as loss of cBZR occurs in association with hemodynamic deterioration without overt episode of stroke suggests that selective neuronal damage may occur in association with hemodynamic compromise in patients whose atherosclerotic ICA or MCA disease is asymptomatic. To test this hypothesis, we measured cBZR using PET and 11C-FMZ in 79 patients with asymptomatic atherosclerotic ICA or MCA disease and no cortical infarction (Yamauchi et al. 2011b). 3D-SSP was used to calculate the BZR index, a measure of abnormally decreased cBZR in the cerebral cortex within the MCA distribution. The BZR index was abnormal (>5.84) in 71 % of the asymptomatic patients compared to healthy control subjects. This abnormality was mild compared with that previously reported for symptomatic patients (the BZR index; 16.0 ± 14.5 vs. 48.2 ± 54.1). Multiple regression analysis showed the BZR index to be positively correlated with the value of OEF, with the presence of silent subcortical infarcts and with the presence of ischemic heart disease, which suggested that hemodynamic compromise is associated with selective neuronal damage in asymptomatic patients. Hemodynamic compromise may cause silent cortical neuronal damage in asymptomatic patients as well. Patients at high risk of stroke may have already suffered ischemic cerebral damage at the time their asymptomatic ICA or MCA disease is discovered. Therapeutic strategies to prevent neuronal damage, including vascular reconstruction surgery, may be called for in asymptomatic patients with chronic hemodynamic compromise. On the other hand, the BZR index was negatively correlated with the treatment of hypertension with angiotensin receptor blockers (ARBs). ARBs were associated with preservation of cortical cBZR. Several experimental studies have shown that ARBs can attenuate ischemic neuronal injury through various mechanisms, including decreased oxidative stress and improved arterial compliance (Mogi and Horiuchi 2009). ARBs might have beneficial effects against neuronal damage in atherosclerotic ICA or MCA disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree