Imaging of Intensive Care Patients after Abdominal Surgery 5 Complications of Specific Operations Several different abdominal drainage systems are used postoperatively or in the setting of interventional procedures. Besides carrying out interventional procedures, the tasks of the radiologist include checking the position of drainage systems and detecting possible complications (e. g., perforation). The two main types of drain are used: overflow drains and suction drains (Table 5.1). In an “open” system, secretions drain into the wound dressing or into a collection bag. This type is illustrated by the Easy-Flow drain (a soft silicone tube with a capillary action) and the Penrose drain (contains a gauze wick that also drains by capillary action). In a “closed” system, fluids drain into a collection bag equipped with a valve and stopcock. Examples are the Robinson drain (silicone drain with a round cross-section and sideholes) and Honig drain (similar design made of soft latex material). A Redon drain (suction drain) employs a vacuum to draw fluid from the body. A Jackson-Pratt drain (bulb drain) is a suction drainage device used primarily in neurosurgery. Suction–irrigation drains are used in patients with a body cavity infection (e. g., peritonitis or empyema of the knee joint). Typically, two separate lines are used for suction and irrigation. The Völcker drain is a biliary drain that is advanced percutaneously through the bowel following hepaticojejunostomy. The Tenckhoff catheter is a drainage device used in chronic abdominal peritoneal dialysis (CAPD).
Abdominal Drainage
B. Partik and P. Pokieser
Types of Abdominal Drains and their Applications
Classification by Drain Type
Overflow Drain
Suction Drains
Special Drains
Type of drain | Examples | Application |
Overflow drains |
|
|
| Penrose drain, Easy-Flow drain | Drainage of anastomoses |
| Robinson drain Honig drain | Bloody drainage Septic surgery |
Suction drains | Redon drain Suction–irrigation drains | Subcutaneous wound drainage Peritonitis |
Special drains | Völcker drain Tenckhoff catheter | Percutaneous biliary drainage Chronic abdominal peritoneal dialysis |
Classification by Function
Bloody Drainage
Some drains are placed to allow the prompt detection of bleeding and provide for drainage of blood and lymph. The Robinson drain and Honig drain are most commonly used. These drains are usually removed on the second postoperative day.
Drainage of Anastomoses
Drains are placed close to surgical anastomoses to allow for prompt drainage of anastomotic leaks. The absence of drainage confirms that the anastomosis is secure. These drains are generally removed on the sixth postoperative day. Not all anastomoses are drained. For example, the mobility of a small bowel anastomosis would limit the options for tube placement, whereas a deeply placed rectal anastomosis would be amenable to drainage. Most anastomotic drains are made of soft material (Penrose, Easy-Flow).
Drainage in Septic Surgery
Honig and Robinson drains are preferred for the drainage of abscesses.
Dwell Time
The timing of drain removal depends on the volume and quality of the drainage. With a pancreatic anastomosis, for example, the drain should be removed when the output falls below 50 mL/day. In the case of an infection, the drain should be left in situ for a longer time.
Feeding Tubes
Small-bore feeding tubes for enteral nutrition have a diameter of 6–8F (1F = 0.3 mm) while large-bore tubes may be as large as 18F. Larger tubes are marked with radio-paque stripes to aid localization. Enteral feeding in intensive care unit (ICU) patients can be done through a nasoenteral tube (gastric, duodenal, or jejunal), percutaneous gastrostomy, or catheter jejunostomy.
Stomach Tube
A correctly placed stomach tube descends through the left upper quadrant of the abdomen. Possible complications are perforation and malposition (e. g., intratracheal or intrapulmonary insertion, placed too high in the esophagus; see also Catheters and Monitoring Devices in Chapter 2). The risk of reflux and aspiration is increased in ventilated, elderly, or unconscious patients. Note that sideholes are often located up to 10 cm from the distal end of the tube.
Duodenal Tube
The tip of the duodenal tube is typically located to the right of the midline in the epigastrium. Positioning is often aided by gastroscopy. A duodenal tube significantly reduces the risk of aspiration compared with a nasogastric tube.
Percutaneous Endoscopic Gastronomy Tube
The percutaneous endoscopic gastronomy (PEG) tube is introduced by percutaneous endoscopy or interventional radiology. Possible complications are clogging of the tube, dislodgement, intraperitoneal malposition, or infection at the incision site.
Catheter Jejunostomy
A jejunostomy tube is placed surgically, usually as part of a larger abdominal operation (gastrectomy, esophageal surgery). The tube may remain in place for up to 3 months. Possible complications are catheter obstruction, accidental removal, intra-abdominal displacement, mechanical bowel obstruction, or local wound infection.
Imaging
Often only a conventional radiograph is taken to assess the position of a stomach tube or duodenal tube. In doubtful cases an additional view is obtained after the instillation of a water-soluble, nonionic, iodinated contrast medium. This material is routinely instilled for the radiographic localization of a PEG tube or catheter jejunostomy. Feeding may commence immediately, once correct placement has been confirmed. If a complication is suspected, further evaluation by CT is indicated.
Biliary Drainage
There is controversy as to the need for biliary drainage in cases where the common bile duct has been opened. Most authors advocate drainage because the rate of complications is higher without it.
The Kehr T-drain is widely used for biliary drainage. Straight, single-lumen tubes with side openings can also be used. The drain is removed between 2 and 3 weeks postoperatively.
The common duct can be stented with a tube to maintain biliary drainage as a palliative measure for stricture or stenosis. A biliary stent can be placed by percutaneous transhepatic cholangiography, the endoscopic retrograde technique, or by nasobiliary catheterization.
Examination Technique
T-tube cholangiography is usually performed on the sixth or seventh postoperative day. A plain radiograph is taken, and then contrast medium is instilled by manual retrograde injection through the T-tube to opacify the biliary tract including the intrahepatic ducts. Approximately 10 mL of undiluted, water-soluble, nonionic, iodinated contrast medium should be used for the detection of peripheral leaks.
Complications
Possible complications are residual stones in the common duct, obstruction of the T-drain by inspissated biliary fluid, and blood clots or strictures of the bile duct at the insertion site of the T-drain, which may necessitate reoperation.
Patients occasionally develop postoperative external fistulas through the skin or internal fistulas that communicate with the duodenum, colon, or stomach. Most of these fistulas will close spontaneously and without sequelae in the absence of a distal biliary tract obstruction or biliary tract injury.
Perforation during stenting of the common duct or papilla may lead to cholascos (escape of bile into the peritoneal cavity) with subsequent biliary peritonitis.
Other complications of biliary tract surgery include hemorrhage, suture-line leak, and subhepatic fluid collections including abscess or empyema formation, which are investigated by CT (see sections below on Biliary Tract Surgery and Cholecystectomy).
Urinary Tract Drainage
Percutaneous Nephrostomy and Ureteral Stenting
Percutaneous nephrostomy (PCN) is the procedure of choice for the decompression of hydronephrosis or for establishment of access for nephroscopy, lithotripsy, or antegrade ureteral stenting. Various techniques employ a fine percutaneous needle to catheterize the renal pelvis through a calyx under local anesthesia (using sono-graphic and/or fluoroscopic guidance). The tract is dilated, and a drainage catheter (12–16F) or ureteral stent is introduced.
Complications. Potential complications are a bleeding arteriovenous (AV) fistula and the development of a false aneurysm or urinoma. Suspected complications should be investigated by ultrasonography (duplex if necessary) and by CT.
Bladder Catheterization
Catheters can be introduced into the bladder by the suprapubic or transurethral route. Bladder catheterization is essential for monitoring and adjusting the fluid balance in ICU patients.
Transurethral catheterization is technically easy to perform but carries a higher risk of infection and late sequelae (strictures, epididymitis) than suprapubic bladder aspiration. The latter procedure is done on a dis-tended bladder under ultrasound guidance using a special disposable instrument set.
Correct catheter placement can be confirmed by fluoroscopically guided contrast instillation. Imaging should be done in two planes (supine and lateral oblique projections).
Complications. Any suspected complications should be investigated by a CT study that includes a delayed series 10 min after IV contrast administration, or by ultrasound scanning of the well-distended bladder. Contrast medium should be diluted 1:20 for intravesical instillation.
Normal Postoperative Findings
S. Kreuzer and C. Schaefer-Prokop
Consistently occurring postoperative findings require differentiation from actual complications. This distinction is aided by noting the typical timeline of specific complications and the types of complication that are most likely to arise after certain operations (Table 5.2):
 The most frequent complication during the early postoperative period (days 1–2) is bleeding.
The most frequent complication during the early postoperative period (days 1–2) is bleeding.
 Infections are most common during the late postoperative period (days 3–10). The causative dehiscence of a bowel anastomosis typically occurs on day 6 or 7. Other causes of infection or sepsis are retention (abscess), infected hematomas, and the leakage of bile.
Infections are most common during the late postoperative period (days 3–10). The causative dehiscence of a bowel anastomosis typically occurs on day 6 or 7. Other causes of infection or sepsis are retention (abscess), infected hematomas, and the leakage of bile.
 Postoperative mechanical bowel obstruction most commonly occurs on day 4. Intestinal peristalsis will generally resume spontaneously by the third postoperative day.
Postoperative mechanical bowel obstruction most commonly occurs on day 4. Intestinal peristalsis will generally resume spontaneously by the third postoperative day.
Postoperative (Physiologic) Fluid Collections
Postoperative intra-abdominal fluid collections do not always signify an abscess or hemorrhage. Fluid retention lasts until postoperative day 4 in ca. 20% of patients, day 8 in 6%, and day 12 in ca. 2%. Typically these (physiologic) fluid collections are crescent-shaped, their shape conforms to surrounding structures, and they occur in preexisting spaces or recesses, where they become spontaneously reabsorbed by the peritoneum.
Postoperative (Physiologic) Intestinal Atony
Reflex or pharmacologically induced gastrointestinal atony (functional or adynamic ileus) consistently occurs during the first two postoperative days, especially after operations that involve the bowel. The paralysis results from premedication and general anesthesia and from the surgical procedure itself (intraoperative therapy, autonomic response).
Usually, peristalsis does not resume until the third postoperative day. The resumption of peristalsis usually occurs spontaneously. Thus, bowel distention is consistently encountered during the immediate postoperative period. It can significantly impede sonographic examination and may increase abdominal circumference in a way that mimics postoperative bleeding.
Imaging
The bowel appears moderately dilated with little or no peristalsis. There is no abrupt cutoff of the gas column, but significant gaseous distention is common. In cases of nonobstructive paralysis, the dilatation involves the small bowel and the ascending colon as far as the hepatic flexure. The rest of the colon has a normal caliber.
Postoperative Pneumoperitoneum
Free intra-abdominal air is a normal finding in the immediate postoperative period. It is highly variable in its duration and extent, depending on patient habitus and the nature of the surgery. The prevalence of postoperative free-air decreases with time after surgery, declining from 44% during the first 3 days to 31% by days 4–7.
The quantity of free air is highly variable. Air following a laparoscopy (insufflated CO2) is absorbed more rapidly than after other types of surgery. Free air has been found with greater frequency and duration in thin patients, male patients, and patients with indwelling drains. Otherwise no correlation has been found between the type of surgery, patient age, and the extent of postoperative pneumoperitoneum.
Note that even patients with an anastomotic leak will not necessarily have free air at CT examination, so the exclusion of a pneumoperitoneum does not prove that the gastrointestinal tract is intact.
Postoperative Complications
C. Schaefer-Prokop, S. Kreuzer, T. Sautner, and W. Schima
Diagnostic Strategy and Examination Technique
The imaging strategy will depend upon whether we must evaluate:
 an extubated, mobilized patient who is a good candidate for abdominal radiography and radiographic follow-ups, or
an extubated, mobilized patient who is a good candidate for abdominal radiography and radiographic follow-ups, or
 an ICU patient who is still on mechanical ventilation; radiographs are technically difficult to perform and are rarely diagnostic in this subgroup, and primary sectional imaging studies (ultrasonography and CT) are preferred.
an ICU patient who is still on mechanical ventilation; radiographs are technically difficult to perform and are rarely diagnostic in this subgroup, and primary sectional imaging studies (ultrasonography and CT) are preferred.
Plain Abdominal Radiograph
A plain abdominal radiograph is indicated in patients with symptoms of ileus and suspicion of a (new) pneumoperitoneum. It is helpful in identifying residual contrast medium from prior examinations and differentiating it from contrast extravasation.
Ideally, plain abdominal radiographs should be taken in two planes, although this is usually not practical in the ICU:
 Initial anteroposterior (AP) supine radiograph using a grid cassette (35 × 43 cm) and low kilovoltage (70 kV) demonstrates organs and soft tissues.
Initial anteroposterior (AP) supine radiograph using a grid cassette (35 × 43 cm) and low kilovoltage (70 kV) demonstrates organs and soft tissues.
 Left lateral decubitus AP radiograph using a grid cassette (35 × 43 cm) and high kilovoltage (125 kV) detects air–fluid levels, assesses intraluminal gas distribution, and detects free air and atypical gas collections (pneumatosis, pneumobilia, etc.). As an alternative, the second view may be a supine cross-table projection with a laterally mounted cassette.
Left lateral decubitus AP radiograph using a grid cassette (35 × 43 cm) and high kilovoltage (125 kV) detects air–fluid levels, assesses intraluminal gas distribution, and detects free air and atypical gas collections (pneumatosis, pneumobilia, etc.). As an alternative, the second view may be a supine cross-table projection with a laterally mounted cassette.
Ultrasonography
Owing to its convenience and availability, ultrasonography is the primary imaging study for the detection of free fluid or suspected fluid collections.
Even small amounts of intra-abdominal free fluid can be detected with high sensitivity (> 90%). Sites of predilection for fluid collections are the Morison pouch (hepatorenal recess) and the lesser pelvis.
As to the limitations of ultrasonography, (small) fluid collections due to leaks cannot be distinguished qualitatively from postoperative reactive or lymphogenous fluid collections that are still within normal limits, or from preexisting ascites. Intraluminal bowel hemorrhages are also difficult to detect on ultrasound scans.
An important advantage of ultrasonography (as an adjunct to CT) is its ability to distinguish an abscess from a fluid-filled bowel loop based on the real-time evaluation of peristalsis.
Radiography
Radiographic follow-ups after oral contrast administration (water-soluble Gastrografin) for detecting leaks or obstructions are rarely practiced nowadays, especially in ICU patients.
Computed Tomography
CT is the imaging modality of choice for almost all postoperative complications (bleeding, abscess, obstruction, leakage).
Oral contrast administration? The bowel can be opacified in the subacute stage with ca. 1 L of a diluted water-soluble contrast medium, administered orally, to distinguish fluid-filled bowel loops from extraintestinal collections. Oral contrast administration is often not possible during the acute stage and will only cause needless delay.
In principle, however, an abscess or bowel obstruction can be diagnosed even without IV contrast. Thus, a contraindication to contrast medium (renal failure) does not justify withholding a CT examination.
CT or ultrasonography? CT is preferred for the diagnosis of intra-abdominal abscess and for locating the focus in postoperative sepsis. Although ultrasound scanning is advantageous in its rapid availability (e. g., in the supine ICU patient), its technical limitations and frequent unfavorable acoustic conditions favor early evaluation by CT. Both modalities can also be used successively and in a complementary fashion.
While intrasplenic, intrahepatic, subhepatic, and subphrenic abscesses can usually be diagnosed with ultra-sonography, CT is better for detecting retroperitoneal abscesses and abscesses located between bowel loops or within the lesser pelvis.
Finally, ultrasonography and CT are equally useful in directing diagnostic or therapeutic needle aspiration (and drainage) and for distinguishing an abscess from noninfectious lesions such as pancreatic pseudocyst, hematoma, bilioma, seroma, aseptic necrosis, postoperative fluid collection, or an unexpected tumor.
CT densitometry is better than ultrasonography for differentiating among the fluid components of normal postoperative transudate, proteinaceous exudate, and hemorrhage. Results are compromised, however, by overlaps of measured attenuation values and mixtures of different components (see Table 5.3).
Intraluminal
|
Intraperitoneal
|
Nonspecific sites
|
During the first 24 hours
|
Secondary bleeding (days 7–10)
|
Causes unrelated to the surgical site
|
Angiography
Angiography or CT? Before fast CT scanners became available, selective angiography was the mainstay for identifying the source and cause of acute bleeding. The advantage of CT is its immediate and high diagnostic accuracy. But most studies require large amounts of contrast medium (up to 150 mL), which may be a limiting factor in acute or postoperative patients with impaired renal function, especially since postcontrast CT is often followed by angiography. This should be a minor handicap in cases where imaging is followed by definitive surgical treatment. The advantage of (super)selective angiography is that it provides access for immediate interventional therapy.
Angiography can detect continuous, active bleeding at rates higher than 0.5 mL/min. It should be noted, however, that not all bleeds are continuous and many sites bleed intermittently. This explains the frequently negative results of CT or angiography in patients with clinical manifestations of gastrointestinal bleeding (melena, etc.; see also the section Acute Gastrointestinal Bleeding in Chapter 4, p. 129).
Sequence of vessel opacification. Often the bleeding site has already been identified, at least with high probability, during previous endoscopy, allowing angiography to be initiated in a specific vascular region (e. g., a bleeding site in the small bowel). The bowel should be effectively immobilized (metoclopramide, glucagon) to create ideal conditions for subtraction views. Otherwise each series should be performed with sufficient contrast medium to allow for satisfactory image interpretation, even in un-subtracted views.
If the source of the hemorrhage cannot be located, all the mesenteric vessels should be selectively visualized in a designated sequence, starting with the inferior mesenteric artery before it is obscured by contrast excretion into the bladder (the imaging sequence does not matter if the bladder has been catheterized). The celiac trunk and superior mesenteric artery are visualized next. Survey angiograms are not sufficient, especially in the celiac trunk and inferior mesenteric artery, and should be supplemented by superselective series of, say, the left gastric artery, gastroduodenal or ileal artery, and jejunal branches, so that the source of the bleeding can be identified with high confidence. Provocation is often done at this stage to locate an intermittent hemorrhage that is still occult. Provocation with intra-arterial heparin or Actilyse is rarely practiced today.
Treatment. Ideally, angiography will demonstrate the extravasation of contrast medium into the bowel lumen. Contrast pooling is another definite sign, and both will document the location of the bleeding site. With both upper and lower gastrointestinal bleeding, the hemorrhagic area is selectively catheterized with a coaxial microcatheter and is embolized with coils as close as possible to the bowel wall.
Pharmacologic therapy (vasoconstriction by IA vasopressin infusion) is mainly of historical interest but may still be tried in rare cases where a bleeding site cannot be identified.
Scintigraphy
Radionuclide imaging techniques have become much less important owing to the broad and rapid availability of sectional imaging modalities. Only the technetium-labeled red blood cell scan still has a significant role in detecting intraluminal bleeding at rates up to 0.1 mL/min. As with other modalities, intermittent bleeding is frequently occult on radionuclide scans. Unlike conventional and CT angiography, scintigraphy is not useful for acute diagnosis.
Postoperative Bleeding
(Primary) bleeding is classified as an immediate postoperative complication. Secondary bleeding is a delayed complication that occurs in the setting of a disorder unrelated to the primary operation, an undetected lesion, or secondary vascular erosion due to inflammation (Table 5.3).
Postoperative bleeding may be intraluminal, usually in the bowel, or may spread freely within the intra- or extraperitoneal space.
Pathogenesis
Primary bleeding occurs during the first 24 hours after the operation. It may be caused by the inadequate ligation or cauterization of blood vessels.
Secondary bleeding usually occurs between postoperative days 7 and 10. It may result from infection causing mucosal damage at the anastomotic site, the erosion of large vessels due to abscess formation, vascular injury caused by drains, or bleeding granulation tissue. Secondary bleeding is more common in patients with inflammatory bowel disease (steroid therapy).
Bleeding due to coagulation disorders or anticoagulant medication is rare in patients who have been adequately prepared. The differential diagnosis should include bleeding stress ulcers distant from the primary surgical site and bleeding lesions that were not identified or considered during the operation (e. g., bleeding from angiodysplasia of the ascending colon following a sigmoid colon resection for diverticulosis).
Clinical Aspects
Patients with postoperative bleeding have typical clinical manifestations. Shock symptoms with a fall in blood pressure accompanied by tachycardia, sweating, and a falling hematocrit or low hemoglobin level on blood tests are suggestive of a hemorrhagic complication. Drainage is monitored to check for bleeding from deep drains or direct bleeding from the surgical wound.
The differential diagnosis should always include the possibility of intra-abdominal bleeding, even after operations without a high bleeding risk.
Imaging
Ultrasonography
The differentiation of “normal” postoperative free-fluid from incipient bleeding or complications may be difficult in any given case. A fluid collection with a spherical or ellipsoidal shape and associated mass effect is suspicious for an abnormal cause (when combined with clinical findings).
Computed Tomography
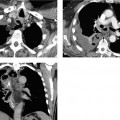
The CT findings of free intraperitoneal blood depend strongly on the age and extent of the hemorrhage (Fig. 5.1). Measured attenuation values are helpful but not definitive (Table 5.4).
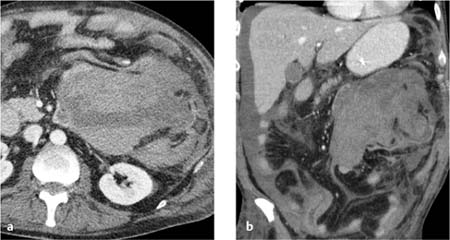
Mesenteric hematomas. These collections appear on CT as ellipsoidal structures of soft-tissue attenuation (Fig. 5.2). Acute, freshly clotted hematomas have attenuation values in the range of 20–40 HU, but these values decline over a period of 1–2 weeks due to the breakdown of hemoglobin. The initially homogeneous structure of the hematoma is transformed into an inhomogeneous “mass.” This makes it difficult to distinguish a chronic hematoma from an abscess with attenuation values of 15–35 HU. As the hematoma continues to age, however, it becomes progressively smaller and finally assumes the size and density (12–25 HU) of a seroma.
Fig. 5.1 a–c Variable CT appearance of hematomas.
a Hematoma appearing as bloody ascites (note the density and sedimentation effect).
b Hematoma appearing as a loculated, inhomogeneous, hyperdense fluid collection following multiple slow bleeds.
c Hematoma appearing as a homogeneous fluid collection with a layer of sedimentation after a single profuse bleed.
Cause | Hounsfield units |
Acute hematoma | > 30 HU (caution: > 20 HU in anemia; depends on hematocrit) |
Subacute hematoma with clotted blood | 40–60 HU. Similar HU values in infection (30–40) |
Arterial extravasate | 80–130 HU (surrounded by lower-density hematoma); local values up to 170 HU at active bleeding site |
Transudate | 5–20 HU |
Exudate | 20–40 HU |
Ascites with significant bleeding | > 30 HU |
Seroma | 10–25 HU |
Bilioma | 0–10 HU; slightly higher with infection (10–15 HU) |
Abscess (pus) | 20–35 HU (30–40) |
Free hemorrhage. Even with CT, it can be difficult to distinguish free intraperitoneal bleeding from an abscess. CT will sometimes show fine sedimentation in the posterior dependent recesses as evidence of bleeding in preexisting ascites.
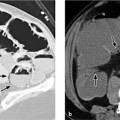
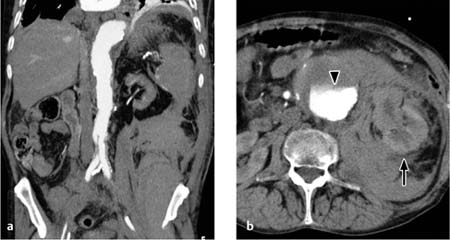
Retroperitoneal hematomas. A definite advantage of CT is its ability to evaluate the retroperitoneum and detect retroperitoneal hematomas (e. g., following aortic surgery or due to a coagulation disorder). Retroperitoneal hematomas appear on CT as patchy, relatively hyperdense masses (> 30 HU) that displace normal retroperitoneal structures (Fig. 5.3).
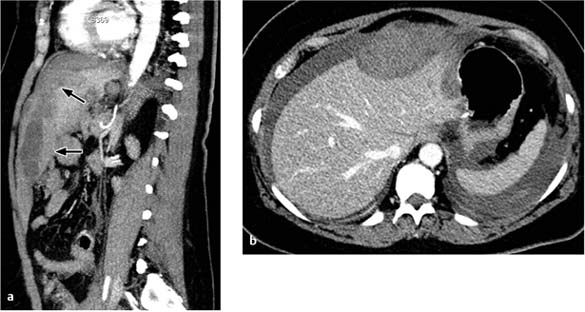
Intraparenchymal hematomas. These collections appear as crescent-shaped masses that compress the organ parenchyma (spleen, liver) when subcapsular (Fig. 5.4).
Abdominal wall hematomas. Abdominal wall hematomas are not uncommon and are demonstrated equally well by ultrasonography and CT, although ultrasound scanning is often more difficult postoperatively and CT provides a better overview of findings.
Postoperative Sepsis and Focus Identification
Sepsis describes a systemic response to an infectious focus located in the body (not necessarily intra-abdominal). Any monitoring change noted in the postoperative patient may be an initial sign of early sepsis: cardiovascular parameters, blood gas analysis, ventilation, laboratory values, body temperature, or general status.
Common findings in postoperative sepsis are paralytic ileus with distention of the small and large bowel and diffuse subcutaneous edema. The patient is febrile and, when conscious, complains of nausea and vomiting. A precipitous fall in white blood count (e. g., from 13 000 to 3000) is indicative of gram-negative sepsis.
Fig. 5.2 a, b Extensive intraperitoneal (mesenteric) hematoma.
Shown here with a sedimentation layer and bloody ascites.
Fig. 5.3 a, b Extensive retroperitoneal bleeding from the aorta with displacement of the left kidney (arrow).
Note in b the sedimentation of contrast medium in the aneurysmatic aorta (arrowhead) due to impaired left ventricular function.
Fig. 5.4 a, b Subcapsular hematoma in the liver.
A subcapsular hematoma is impressing on the (soft) liver tissue. Patient presented with a sudden decrease of hemoglobin level 8 days after partial gastrectomy.
Postoperative days | Abdominal complications |
Days 1–2 |
|
Days 2–3 |
|
Days 3–6 |
|
Days 7–14 |
|
“Four P’s” should be considered in the differential diagnosis of sepsis in ICU patients: pneumonia, peritonitis, phlebitis, and pyelitis. The development of septic complications after surgery follows a classic timeline (Table 5.5). Awareness of this timeline can be helpful in narrowing the differential diagnosis.
Peritonitis
Peritonitis is defined as an inflammatory reaction of the peritoneum to pathogenic stimuli such as bacteria, viruses, and chemical agents.
Classifications
Primary peritonitis (hematogenous, lymphogenous, or luminal invasion/contamination) is distinguished from secondary peritonitis due to a perforated hollow viscus and from tertiary or persistent peritonitis. While secondary peritonitis is treated by surgical eradication of the focus and pharmacologic therapy serves only as an adjunct, the causal treatment for primary peritonitis is antibiotic pharmacotherapy.
Surgical treatment is appropriate for primary peritonitis only in cases that have not responded to antibiotics. Therapeutic options are debridement and copious irrigation of the abdominal cavity to reduce microorganism counts.
For the prevention of persistent “tertiary” peritonitis and intra-abdominal abscess formation (ca. 20% incidence after secondary peritonitis), it is generally agreed that eradication of the focus should be followed by additional surgical measures based on the principle of “need-oriented relaparotomy” (= reoperation prompted by a deterioration of the patient’s general condition).
Postoperative peritonitis, which most commonly results from anastomotic leak, affects a patient that is already traumatized by the previous operation and is subject to an “acute-phase response” (with systemic activation of mediators). Today it is believed that the acute-phase 20% incidence after secondary peritonitis), it is generally agreed that eradication of the focus should be followed by additional surgical measures based on the principle of “need-oriented relaparotomy” (= reoperation prompted by a deterioration of the patient’s general condition). Response combined with the infectious “second hit” may initiate the development of multiorgan dysfunction syndrome (MODS). The mortality rate of this condition, at 50%, is substantially higher than that of a primary hollow viscus perforation.
The routine clinical description of “(single) quadrant peritonitis” or “multiquadrant peritonitis” is based on a gross subdivision of the abdomen into four quadrants and does not follow predefined anatomical boundaries.
Clinical Aspects
The main clinical symptoms of peritonitis are abdominal pain and tenderness, rigidity, aperistalsis (silent abdomen), and systemic disease manifestations. They are accompanied by circulatory symptoms (fever, chills, tachycardia and tachypnea, and eventual signs of shock). The severity of the symptoms correlates with the cause and mortality risk (Table 5.6).
The symptoms of peritonitis may be masked in postoperative or immunocompromised patients and in very young or very old patients. The diagnostic challenge is not to diagnose peritonitis but to identify its cause.
Severity | Cause | Mortality |
Mild |
| <10% |
Moderate |
| <20% |
Severe |
| 20–80% |
Imaging
Plain Abdominal Radiograph
Due to the frequency of perforation-induced peritonitis, a diagnosis of peritonitis should be considered whenever a pneumoperitoneum is found. Abdominal radiographs show generalized intestinal atony and distention with fluid levels in the small and large bowel. Specific signs of peritonitis are not seen. Displacement and obvious asymmetry of bowel loops is suggestive of an abscess.
Ultrasonography
Ultrasound reveals atonic, fluid-filled bowel loops. Free fluid is detected at sites of predilection. Attention is given to the morphology of the gallbladder (caution: cholecystitis) and to any fluid collections that are suspicious for an abscess (see below).
Computed Tomography
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Overflow drains consist of open and closed systems. Suction drains employ a vacuum to draw fluid from the body.
Overflow drains consist of open and closed systems. Suction drains employ a vacuum to draw fluid from the body. Open systems
Open systems Closed systems
Closed systems The timing of drain removal depends on the volume and quality of the output.
The timing of drain removal depends on the volume and quality of the output. A water-soluble, nonionic, iodinated contrast medium is instilled for the radio-graphic localization of a PEG tube or catheter jejunostomy tube.
A water-soluble, nonionic, iodinated contrast medium is instilled for the radio-graphic localization of a PEG tube or catheter jejunostomy tube. Avoid injecting air bubbles into the T-drain, as they may be mistaken for stones.
Avoid injecting air bubbles into the T-drain, as they may be mistaken for stones. Transurethral catheterization is easy to perform but carries a higher risk of infection than suprapubic bladder aspiration.
Transurethral catheterization is easy to perform but carries a higher risk of infection than suprapubic bladder aspiration. Resumption of peristalsis generally occurs spontaneously by the third postoperative day.
Resumption of peristalsis generally occurs spontaneously by the third postoperative day. Postoperative intra-abdominal fluid collections are not necessarily an abscess or hemorrhage.
Postoperative intra-abdominal fluid collections are not necessarily an abscess or hemorrhage.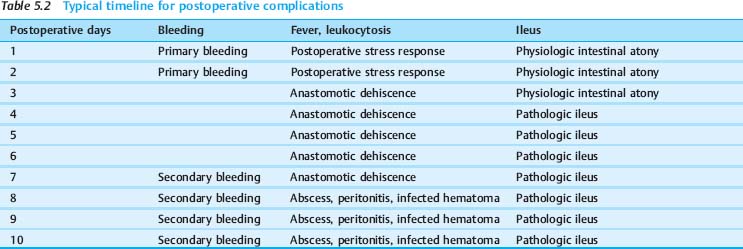
 Small amounts of postoperative free-air before day 18 do not signify an anastomotic leak or perforation, but increasing or voluminous free air is suspicious.
Small amounts of postoperative free-air before day 18 do not signify an anastomotic leak or perforation, but increasing or voluminous free air is suspicious. Fluid collections in the hepatorenal recess and lesser pelvis are the earliest postoperative collections that are detectable by ultrasound scanning.
Fluid collections in the hepatorenal recess and lesser pelvis are the earliest postoperative collections that are detectable by ultrasound scanning. Angiographic detection requires continuous, active bleeding at a rate higher than 0.5 mL/min.
Angiographic detection requires continuous, active bleeding at a rate higher than 0.5 mL/min. Intrasplenic, intrahepatic, subhepatic, and subphrenic abscesses can usually be diagnosed with ultrasonography. CT is better for detecting retroperitoneal abscesses and abscesses located between bowel loops or in the lesser pelvis.
Intrasplenic, intrahepatic, subhepatic, and subphrenic abscesses can usually be diagnosed with ultrasonography. CT is better for detecting retroperitoneal abscesses and abscesses located between bowel loops or in the lesser pelvis. Gastroduodenal ulcer and erosions
Gastroduodenal ulcer and erosions Sloughing of mucosa
Sloughing of mucosa Vascular or organ injury
Vascular or organ injury Loose or slipped vascular ligature
Loose or slipped vascular ligature Erosion by abscess
Erosion by abscess Second or undetected focus
Second or undetected focus Hemorrhagic diathesis
Hemorrhagic diathesis Inadequate hemostasis
Inadequate hemostasis Slipped ligature
Slipped ligature Abscess
Abscess Anastomotic infection
Anastomotic infection Injury by drains
Injury by drains Granulation tissue
Granulation tissue Stress ulcer
Stress ulcer Coagulation disorder
Coagulation disorder Occult lesions
Occult lesions Primary bleeding occurs during the first 24 hours after surgery. Secondary bleeding usually occurs between days 7 and 10.
Primary bleeding occurs during the first 24 hours after surgery. Secondary bleeding usually occurs between days 7 and 10.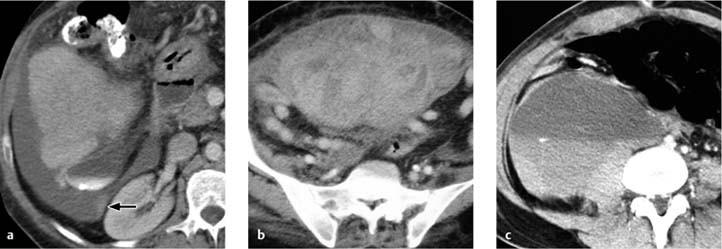
 Postoperative days 1–2 are marked by an inflammatory “stress response” with fever, tachycardia, hyperventilation, and an extravascular and extracellular fluid shift.
Postoperative days 1–2 are marked by an inflammatory “stress response” with fever, tachycardia, hyperventilation, and an extravascular and extracellular fluid shift. Abdominal sepsis is rarely causative; when it is, it is referable to a (major) anastomotic leak with rapid development of peritonitis.
Abdominal sepsis is rarely causative; when it is, it is referable to a (major) anastomotic leak with rapid development of peritonitis. This period is marked by a physiologic reversal of the extravascular fluid shift with increasing diuresis and potential pulmonary edema formation (especially in patients with preexisting heart and/or renal failure).
This period is marked by a physiologic reversal of the extravascular fluid shift with increasing diuresis and potential pulmonary edema formation (especially in patients with preexisting heart and/or renal failure). Fever may be attributable to pneumonia.
Fever may be attributable to pneumonia. Consider anastomotic leak in patients with poor diuresis and no resumption of peristalsis.
Consider anastomotic leak in patients with poor diuresis and no resumption of peristalsis. This is the period in which abdominal sepsis is most common!
This is the period in which abdominal sepsis is most common! Paradoxical diarrhea (do not misinterpret as improvement), intestinal atony, dysuria, cardiopulmonary insufficiency, fever and leukocytosis are symptoms that may be associated with anastomotic leak and peritonitis.
Paradoxical diarrhea (do not misinterpret as improvement), intestinal atony, dysuria, cardiopulmonary insufficiency, fever and leukocytosis are symptoms that may be associated with anastomotic leak and peritonitis. Most postoperative complications during this period are caused by localized abscess formation, usually secondary to an anastomotic leak.
Most postoperative complications during this period are caused by localized abscess formation, usually secondary to an anastomotic leak. The “four P’s” in the differential diagnosis of sepsis in ICU patients are pneumonia, peritonitis, phlebitis, and pyelitis.
The “four P’s” in the differential diagnosis of sepsis in ICU patients are pneumonia, peritonitis, phlebitis, and pyelitis. Primary (spontaneous) peritonitis is distinguished from secondary peritonitis (due to a perforated hollow viscus) and from tertiary (persistent) peritonitis.
Primary (spontaneous) peritonitis is distinguished from secondary peritonitis (due to a perforated hollow viscus) and from tertiary (persistent) peritonitis. Appendicitis
Appendicitis Perforated gastroduodenal ulcer
Perforated gastroduodenal ulcer Acute salpingitis
Acute salpingitis Diverticulitis
Diverticulitis Nonvascular perforation of the small bowel
Nonvascular perforation of the small bowel Gangrenous cholecystitis
Gangrenous cholecystitis Multiple injuries
Multiple injuries Perforation of the large bowel
Perforation of the large bowel Ischemic perforation of the small bowel
Ischemic perforation of the small bowel Postoperative complications
Postoperative complications