Encephalitis is an acute infection of brain parenchyma characterized clinically by fever, headache, and an altered level of consciousness. In the twenty-first century several outbreaks of encephalitis have been reported. Urbanization and encroachment on natural environments, the ease of world travel, and global trade have led to the spread of vectors and viruses.
Encephalitis is an acute infection of brain parenchyma characterized clinically by fever, headache, and an altered level of consciousness . More than 100 viruses are associated with acute central nervous system (CNS) infections . In the twenty-first century several outbreaks of encephalitis have been reported. Urbanization and encroachment on natural environments, the ease of world travel, and global trade have led to the spread of vectors and viruses. Infectious diseases once considered exotic in the developed world are now potential threats everywhere . There has been continued recognition of emerging neurotropic viruses in both developed and developing countries .
The diagnosis in viral encephalitis is based on the synopsis of clinical signs and symptoms, serologic data, cerebrospinal fluid (CSF) analysis, and imaging findings. Neuroimaging constitutes an important component in the diagnosis of underlying infectious agents in the CNS. Because of its higher contrast resolution, MR imaging is more sensitive than CT in detecting inflammatory brain lesions .
Recent MR imaging techniques, such as diffusion-weighted imaging (DWI) and proton MR spectroscopy, provide additional helpful information, but the mainstay of diagnosis remains the detection of viral DNA or serologic markers of specific infectious agents within the CSF .
Herpes simplex virus (HSV) remains the most common cause of viral encephalitis. The recently observed epidemic of the West Nile virus, newly recognized viruses such as the Nipah virus, and the previously unnoticed association of viruses like the human herpes viruses 6 or 7 (HHV 6, HHV 7) or enterovirus 71 with CNS infections point out the need to consider agents other than HSV in acute encephalitis. In immunocompromised patients the spectrum of possible causative agents is even broader . This article focuses on neuroimaging findings of nonherpetic acute viral meningoencephalitis.
Arthropod-borne viral encephalitis
Arthropod-borne viral (arbovirus) infections now are a global health problem. During the past 20 years there has been a dramatic resurgence or emergence of epidemic arboviral diseases affecting both humans and domestic animals. Ecologic changes and human behavior are important in the spread of these infections . The most significant arboviruses causing human illness belong to genera in three viral families: Togaviridae, Flaviviridae, and Bunyaviridae. These arboviruses are transmitted to humans and domestic animals by the bite of infected arthropods. Many arbovirus infections are asymptomatic . Clinical manifestations range from mild febrile illness, which may or may not be accompanied by skin rash and by arthralgia, to severe and often fatal encephalitis or hemorrhagic fever with shock .
Japanese encephalitis, a mosquito-borne flaviviral encephalomyelitis, is endemoepidemic in several countries in East, South-East, and South Asia, affecting more than 50,000 individuals and resulting in more than 10,000 deaths per year . Mortality is about 30%, and survivors often suffer serious long-term morbidity . Up to 50% of those who recover are left with disabling neurologic deficits . Japanese encephalitis is mostly a disease of children . The Japanese encephalitis virus invades the nervous system through the hematogenous route, although subsequent spread of the virus seems to occur along the dendritic axonal processes . Japanese encephalitis usually is a uniphasic illness, but a biphasic pattern has also been described with the appearance of fresh lesions during the second phase at the sites known for their involvement in Japanese encephalitis, suggesting recrudescence of the virus .
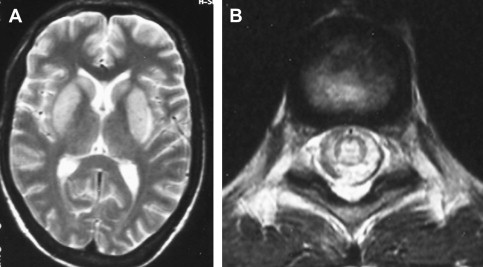
MR imaging is more sensitive and useful than CT in revealing the characteristic radiologic changes in Japanese encephalitis and is recommended as the investigation of first choice in a patient suspected of having Japanese encephalitis . In a recent study conducted in 30 children and 37 adults, cranial CT was abnormal in around 38% of the subjects, whereas MR imaging revealed pathologic changes in 90.6% to 95.5% . Typical MR imaging features consist of either mixed-intensity or hypointense lesions on T1-weighted imaging (T1WI) or hyperintense or mixed-intensity lesions on T2-weighted imaging (T2WI), predominantly in the thalami but also in the basal ganglia, brainstem, cerebellum, and cortical areas ( Fig. 1 ). Some patients present with parkinsonism that probably is caused by a prevailing involvement of the substantia nigra . Medial temporal lobe involvement may be encountered also, characteristically in the posterior part of the hippocampus. Unlike herpes simplex encephalitis, the anterior temporal lobe usually is spared, and insular involvement is rare . In an appropriate clinical setting, the most consistent and characteristic findings in Japanese encephalitis are bilateral thalamic lesions with or without hemorrhagic changes. Hemorrhagic involvement of the thalamus is not reported to correlate with severity of illness or with outcome, however, and possibly is related to a more virulent strain . Both the basal ganglia and thalami can be involved in both Japanese encephalitis and Wilson disease. The posteromedial thalamus, however, characteristically is involved in Japanese encephalitis and spared in Wilson’s disease, which involves anterolateral thalamus . DWI is helpful in characterization of the duration of the lesions and in establishing an early diagnosis of Japanese encephalitis by showing characteristic involvement of bilateral thalami at an early stage . Some authors have reported that co-occurrence of Japanese encephalitis and neurocysticercosis is not just a chance coincidence and that neurocysticercosis apparently predisposes a person to Japanese encephalitis infection . Others found the coexistence of neurocysticercosis and Japanese encephalitis as an incidental finding in areas of high prevalence and endemicity .
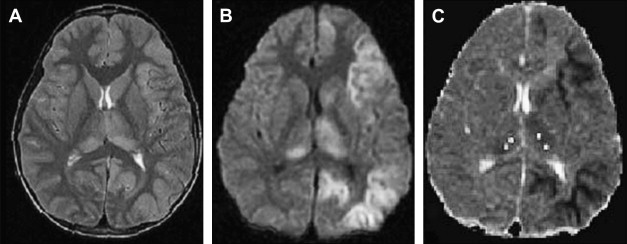
Until the 1999 outbreak in New York City, West Nile virus was found primarily in the eastern hemisphere in Africa, Asia, and the Middle East. The original West Nile virus isolate was reported in Uganda in 1937 . The culicine mosquito acts as the vector for transmitting the virus from birds to humans. Reports also describe transmission through blood transfusion, organ transplantation, and breastfeeding. The incubation period in humans ranges between 3 and 14 days . Most West Nile virus infections are subclinical, with overt disease occurring in 1 of every 100 to 150 WNV-positive individuals . Neurologic involvement is the most significant effect of West Nile virus; symptoms vary from mild weakness to irreversible flaccid paralysis and including a range of cognitive, sensory, and motor deficits involving the central and peripheral nervous systems. Only 35% of patients have complete recovery. The mortality rate is approximately 10% .
As a member of Flaviviridae family, West Nile virus has close antigenic and phylogenetic relationship with Japanese encephalitis, St. Louis encephalitis, and Murray Valley encephalitis. It also shares some of the imaging findings of these entities . There are no reports of a specific abnormality at head CT in West Nile virus encephalitides . Abnormal MR imaging findings in patients who have West Nile virus meningoencephalomyelitis are common but nonspecific. Anatomic areas commonly affected are basal ganglia, thalami, mesial temporal structures, brain stem, and cerebellum. Extremity weakness or flaccid paralysis corresponds to spinal cord/cauda equina abnormalities . Similar to other viral encephalitis, DWI is useful in the early detection of West Nile virus encephalitis at a time when conventional MR imaging is virtually normal ( Fig. 2 ) . It would be impossible in most instances to suggest West Nile virus encephalomyelitis prospectively and based only on MR findings. On the other hand, certain imaging findings such as deep gray matter or mesial temporal lobe involvement can be suggestive of the disease, if additional factors such geographic area, time of the year, and potential exposure to mosquitoes are taken into consideration .
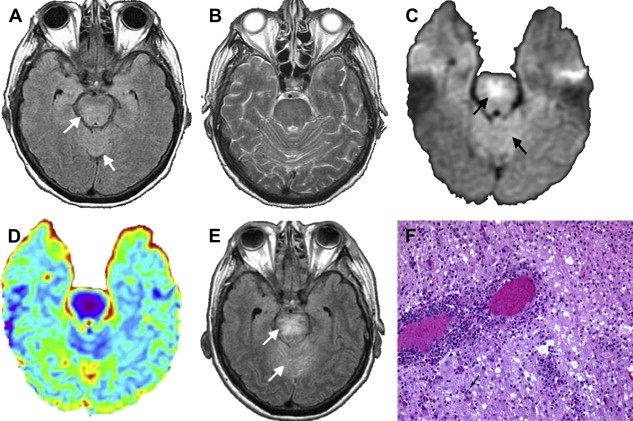
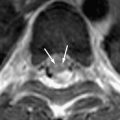
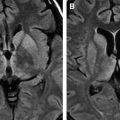
The virus causing St. Louis encephalitis, a member of the Flavivirus (group B) arboviruses, is a common cause of epidemic encephalitis in the eastern and central United States . Although most of the infections are asymptomatic, clinical illness may range from mild febrile illness to severe neuroinvasive disease . The majority of the patients who have St. Louis encephalitis are tremulous, and this feature is particularly characteristic of the disease. To date, there are no reports of a specific abnormality seen in head CT in St. Louis encephalitis. Based on limited reports, MR imaging findings include isolated T2-weighted hyperintensity of the substantia nigra, which correlates with the predominant involvement of the substantia nigra in St. Louis encephalitis that has been reported pathologically . Isolated involvement of substantia nigra also is seen on MR imaging in Japanese encephalitis ( Fig. 3 ) . Involvement of the substantia nigra can also be seen in West Nile virus encephalitis, Eastern equine encephalitis, and tick-borne encephalitis, but involvement is more diffuse in these conditions, with areas of hemorrhage, infarction, edema found in other areas of brain .
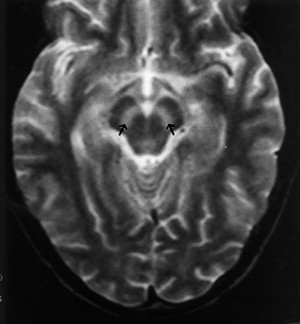
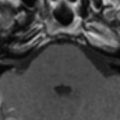
Murray Valley encephalitis, also known as “Australian encephalitis,” belongs to the Japanese encephalitis antigenic complex and is endemic to Australia and Papua New Guinea . The first case that came into notice, however, was from Europe, in a young man who had traveled in Australia . MR imaging demonstrates abnormalities very similar to those in Japanese encephalitis. In a recent report, T2WI shows hyperintense changes within the thalami, red nucleus, substantia nigra, and cervical spinal cord . Tick-borne encephalitis and Eastern equine encephalitis share similar imaging features ( Figs. 4 and 5 ) .
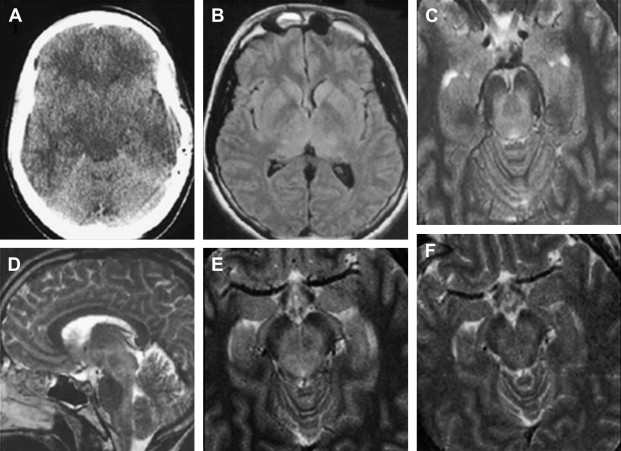
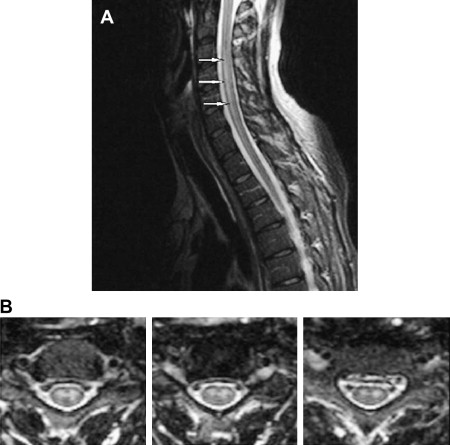
Dengue is one of the most common arboviral infections worldwide and is being recognized increasingly as an important public health problem in Western Pacific regions and in Southeast Asia . It is a single-stranded RNA arbovirus and is classified as a member of the Flaviviridae family . There are four different serologic types, and infection by any of these has been reported to result in dengue fever, dengue hemorrhagic fever, and dengue shock syndrome . It is transmitted by Aedes aegypti .
Classical dengue fever usually is seen in children and young adults . Unlike most other arboviral infections, dengue virus usually is considered a non-neurotropic virus . Neurologic symptoms are considered a rare complication of dengue infection . In recent years, however, it has been noted that this virus also can cause neurologic manifestations . Various neurologic manifestations of dengue infection include headache, seizure, depressed sensorium, behavioral disorders, neck stiffness, delirium, paralysis, cranial nerve palsies, and coma .
Previously, neurologic manifestations were attributed to encephalopathy rather than encephalitis, which is defined as a localized invasion of the CNS . The pathophysiology of the neurologic symptoms in the reported cases have been attributed to cerebral edema, anoxia, hemorrhage, hyponatremia, hepatic failure, microcapillary hemorrhage, and the release of toxic substances . Recent reports, however, have demonstrated a possible neurotropic effect of the virus. Animal studies done in mice showed that the virus could break down the blood–brain barrier leading to CNS invasion .
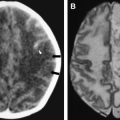
MR imaging is superior to CT in demonstrating most CNS lesions. In most patients who have neurologic symptoms, cerebral edema is predominant finding on imaging studies, although, a few cases do show encephalitis-like changes . Isolated hippocampus involvement also has been reported ( Fig. 6 ) . Intracranial bleeding is an infrequent cause of the neurologic symptoms ( Fig. 7 ) .
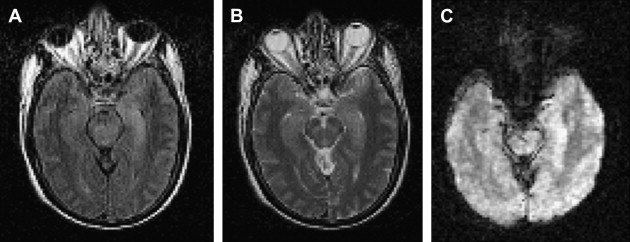
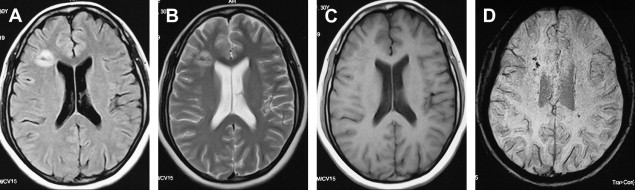
The mortality in cases of dengue encephalitis is increased by the increased incidence of dengue hemorrhagic fever/dengue shock syndrome and not by the encephalitis per se, which usually has a benign course. Therefore it is important for the clinician to be aware of the possibility of dengue infection as a cause of encephalitis .
In summary, because the radiologic findings in various flaviviral encephalitides are similar, imaging features alone are not sufficient to discriminate between these CNS infections and should be interpreted in light of the endemic nature of the disease, clinical context, and virologic studies.
Lyssavirus encephalitis (Rabies)
Rabies is a uniformly fatal disease involving the CNS in humans and other mammals caused by neurotropic RNA viruses in the family Rhabdoviridae, genus Lyssavirus . Transmission to humans is mainly through bites of infected rabid dogs, cats, bats, and other wild animals and, rarely, through inhalation, by contact of infected saliva with an open wound or mucous membrane, and by infected corneal transplants . After inoculation the virus reaches the CNS through retrograde axoplasmic flow . The incubation period of rabies is usually 2 to 8 weeks .
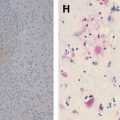
Premortem diagnosis usually is made by performing corneal impression smears, nuchal skin biopsies, or biopsies of the oral or nasal mucosa . Gross pathologic specimens of the brain in rabies show scattered hemorrhages, necrosis, and surrounding edema in the cerebral gray matter, predominantly involving the basal ganglia and the brain stem. Histopathologically, the changes seen in the neurons are necrotic . The pathognomonic histologic feature, the Negri bodies, is present in only 50% to 80% of proven cases .
Classically, human rabies manifests in either encephalitic (furious) or paralytic (dumb) form . Both forms of the disease are invariably fatal, and most patients die within 10 days of the onset of neurologic symptoms . The more common encephalitic form starts with malaise, fever, and paresthesia at the site of the bite followed by classical neurologic symptoms of agitation, hydrophobia, aerophobia, hypersalivation, and seizures . The clinical picture of fully fledged rabies encephalitis is unmistakable. The less common paralytic form, dumb rabies, accounts for 20% of cases and is difficult to diagnose because more than 50% of patients lack the classical symptoms of hydrophobia or aerophobia. It may be confused with other rapidly paralyzing illnesses, and recourse to history, laboratory investigations, and imaging may be crucial for diagnosis .
Literature on neuroimaging in patients who have rabies is sparse. Its rapid and stormy clinical course makes neuroimaging difficult . CT of the brain shows focal or diffuse areas of hypoattenuation in the basal ganglia, periventricular white matter, hippocampus, and brain stem. Pontine hemorrhages also have been reported. Diffuse cerebral edema may be seen in advanced cases .
MR imaging is more sensitive than CT. MR imaging patterns are similar in both forms of human rabies. The predominant involvement of gray matter of the brain and spinal cord ( Fig. 8 ) is a hallmark of rabies and is important in differentiating rabies from acute disseminated encephalomyelitis . On MR imaging, T2-weighted images demonstrate nonenhancing, ill-defined, mild hyperintensity changes in the brain stem, hippocampi, hypothalami, deep and subcortical white matter, and deep and cortical gray matter. With the exception of brachial plexus, which may show enhancement during the prodromal phase of the disease, the hypothalami, brain stem nuclei, spinal cord gray matter, and intradural cervical nerve roots show enhancement only when the patient becomes comatose . Intracranial vasospasm also has been reported as narrowing in the supraclinoid internal carotid and terminal basilar arteries on cerebral angiogram. This vasospasm could to be caused by transient arterial spasm induced by the viral infection .