Eye movement is controlled by ocular motor pathways that encompass supranuclear, nuclear, and infranuclear levels. Lesions affecting certain locations may produce localizing signs that help radiologists focus on specific anatomic regions. Some pathologic conditions, such as aneurysms and meningiomas, have unique imaging characteristics that may preclude unnecessary tissue biopsies. Some conditions are life threatening and require urgent or emergent imaging. MR imaging is the imaging of choice in evaluation of ocular motor palsy, with magnetic resonance angiography or computed tomography angiography indicated in cases of suspected aneurysms or neurovascular conflicts.
Key points
- •
Eye movement is controlled by cortical eye centers, brainstem nuclei, and ocular motor nerves that innervate the extra ocular muscles.
- •
Lesions in certain locations, such as the dorsal brainstem, cavernous sinuses, and superior orbital fissures, may produce localizing signs, which are very helpful for radiologists to focus on those anatomic regions.
- •
Some conditions are life threatening and require urgent or emergent imaging, including pupil-involving oculomotor palsy caused by posterior communicating artery aneurysms, cavernous sinus thrombosis, brainstem stroke, severe trauma, and so forth.
Introduction
To be able to follow a visual target in either vertical or horizontal directions in a slow or rapid manner (pursuit or saccade), both eyes need to move in a conjugate fashion. This movement is controlled by the cerebral cortex, vestibular system, multiple brainstem nuclei, and cranial nerves (CNs) that finally innervate the extraocular muscles, constituting ocular motor pathways. The diseases affecting these pathways may cause diplopia, ocular misalignment, nystagmus, eye movement restriction, and so forth. Clinical presentations can be highly localizing and help alert the radiologist to search for subtle lesions in specific locations. Some of these lesions have imaging characteristics that may help preclude unnecessary biopsies.
Introduction
To be able to follow a visual target in either vertical or horizontal directions in a slow or rapid manner (pursuit or saccade), both eyes need to move in a conjugate fashion. This movement is controlled by the cerebral cortex, vestibular system, multiple brainstem nuclei, and cranial nerves (CNs) that finally innervate the extraocular muscles, constituting ocular motor pathways. The diseases affecting these pathways may cause diplopia, ocular misalignment, nystagmus, eye movement restriction, and so forth. Clinical presentations can be highly localizing and help alert the radiologist to search for subtle lesions in specific locations. Some of these lesions have imaging characteristics that may help preclude unnecessary biopsies.
Normal anatomy
Oculomotor Nerve (Cranial Nerve III)
Oculomotor nuclear complex contains individual motor subnuclei and parasympathetic Edinger–Westphal nuclei (EWN). The motor nuclei are located in the dorsal aspect of the midbrain, ventral to the periaqueductal gray at the level of the superior colliculi ( Fig. 1 ). The EWN are dorsomedial to the motor nuclei. The nerve fascicles pass ventrally through the red nuclei and exit the midbrain at the medial aspect of the cerebral peduncles. The cisternal segments of the nerves are located in the interpeduncular/prepontine cisterns, between the posterior cerebral arteries (PCA) and superior cerebellar arteries (SCA). The nerves then pass medially to the uncus. The parasympathetic fibers are located in the periphery of the nerves; therefore, external compression (eg, posterior communicating aneurysms or uncal herniation) often cause pupil-involving CN III palsy. The short and variable dural cave segments are also surrounded by cerebrospinal fluid (CSF) before the nerves pierce the dura to become the interdural segments in the cavernous sinuses. Within the cavernous sinus, the nerves lie within the lateral walls, superior to CN IV. The nerves then exit anteriorly to enter the superior orbital fissures as foraminal segments. The extraforaminal segments enter the orbits and stay within the annulus of Zinn and intraconal spaces, where they divide into superior and inferior divisions to supply the extra-ocular muscles.
Trochlear Nerve (Cranial Nerve IV)
CN IV is the smallest of the 3 ocular motor nerves, which poses challenges for neuroimaging. The trochlear nuclei are located in the dorsal midbrain, ventral to the periaqueductal gray at the level of the inferior colliculi ( Fig. 2 ). The nerve fascicles decussate within the superior medullary velum and exit the midbrain dorsally. The long cisternal segments course within the quadrigeminal and ambient cisterns, inferior to the tentorial cerebelli free edge. The dural cave segments are typically not visible on imaging. Similar to CN III, the interdural segments (see Fig. 1 D) are within the lateral walls of the cavernous sinuses, between CN III and ophthalmic division of the trigeminal nerve (CN V1). CN IV exits the cavernous sinuses to enter the superior aspect of the superior orbital fissures as the foraminal segments, before reaching the orbit superior to the annulus of Zinn.
Abducens Nerve (Cranial Nerve VI)
The abducens nuclei are located in the dorsomedial aspect of the caudal pons at the level of the facial colliculi, where the fascicular facial nerves loop around to the abducens nuclei ( Fig. 3 ). The CN VI fascicles pass ventrally to exit the pons at the pontomedullary junction. The cisternal segments ascend within the prepontine cistern before entering the dural cave where the nerves remain surrounded by CSF. The interdural segments are divided into 2 subsegments, including the proximal petroclival and distal cavernous subsegments, where the nerves are surrounded by venous channels. The cavernous segments (see Fig. 1 D) are inferolateral to the cavernous internal carotid arteries and medial to the CN V1.
Horizontal Conjugate Gaze
To be able to rapidly follow a visual target to the left side (leftward saccade), the right cortical eye fields (frontal, parietal, and supplementary eye fields) control the left omnipause neurons and the paramedian pontine reticular formation (PPRF). The latter activates the left abducens nucleus and the left lateral rectus via the left CN VI. The left abducens nucleus activates the right medial rectus subnucleus via the crossing medial longitudinal fasciculus (MLF) ( Fig. 4 ). This activation results in simultaneous contraction of the left lateral rectus and the right medial rectus, leading to left conjugate saccade. Therefore, the left abducens nucleus controls the left lateral rectus and the right medial rectus. Lesions of the abducens nucleus cause ipsilateral conjugate gaze palsies, whereas lesions of the MLF result in internuclear ophthalmoplegia (ie, inability to adduct the ipsilateral eye).
Vertical Conjugate Gaze
The pretectal area (area in the midbrain rostral to the red nucleus) houses the vertical conjugate gaze center, which consists mainly of the interstitial nucleus of Cajal (INC), rostral interstitial nucleus of the MLF (riMLF), and the posterior commissure ( Fig. 5 ). For downward gaze, the contralateral INC and the ipsilateral riMLF activate the inferior rectus subnucleus and the trochlear nucleus. Upward gaze occurs when each riMLF activates bilateral INC and ocular motor complex (superior rectus and inferior oblique subnuclei).
Imaging technique and protocol
MR imaging is the technique of choice for depicting the anatomy and disease of the ocular motor pathways. The nuclear and fascicular segments are not typically visualized discretely with 3-T MR imaging but can be localized in the expected location of the brainstem. The cisternal and dural cave segments can be demonstrated on steady-state free precession (SSFP) imaging (eg, constructive interference in the steady state [CISS] or fast imaging employing steady-state acquisition [FIESTA] sequence), which yields heavily T2-weighted images. Acquiring images using thin-cut high-resolution 3-dimensional isotropic sequences enable reconstruction in multiple planes. It is reported that the interdural segment is better demonstrated on contrast-enhanced SSFP. In this sequence, the nonenhancing ocular motor nerves are seen within the lateral wall or inside of the enhancing cavernous sinuses. Computed tomography (CT) and MR imaging are complementary in evaluating the foraminal segments, with CT being valuable in delineating the osseous margins of the foramina, skull base, and orbital walls. Contrast-enhanced T1-weighted MR images help depict pathologic enhancement of the nerves.
Pathology
Nuclear and Fascicular
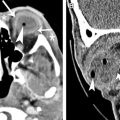
The CN nuclei and fascicles reside in the midbrain and pons; therefore, they can be involved by diseases of the brainstem, including ischemia, neoplasms, demyelination, rhombencephalitis, trauma, neurodegenerative diseases, and metabolic disorders. Because of the close proximity with other brainstem structures, patients typically present with associated brainstem dysfunctions that may produce highly localizing clinical syndromes, such as Weber syndrome ( Fig. 6 ), one-and-a-half syndrome ( Fig. 7 ), and so forth.
Ischemia
Approximately 10% of ischemic strokes involve the brainstem, with the pons more commonly effected than the medulla and midbrain. Isolated midbrain stroke is very rare, but ophthalmoplegia is the second most common symptom after ataxia. Similar to ischemia in other parts of the brain, MR imaging is more sensitive than CT in early detection, showing restricted diffusion (see Fig. 6 ). Pontine infarctions typically do not cross the midline, which follow the distribution of paramedian penetrating branches of the basilar artery (BA). Patients typically present with horizontal gaze palsy. In contrast, infarcts of the midbrain may cross the midline because the arterial supply is not limited to paramedian distribution; patients may present with more complex eye movement disorders. Infarcts of the midbrain can be divided into anteromedial, anterolateral, lateral, and dorsal infarction according to differences in arterial supplies.
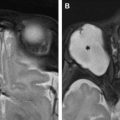
Neoplasms
Brainstem gliomas are the most common primary brainstem neoplasms and are more prevalent in pediatric patients than adult patients. The tumors are classified by MR imaging findings into 4 types: (1) diffusely infiltrative low-grade gliomas ( Fig. 8 A, B ), (2) enhancing focal malignant gliomas (see Fig. 8 C, D), (3) tectal gliomas, and (4) exophytic gliomas. Approximately 36% of adults with the diffusely infiltrative type present with CN VI palsy. Most patients also have other brainstem dysfunction including weakness, dizziness, ataxia, dysphagia, and so forth.