Optic neuropathy involves loss of visual acuity, color vision, and visual field defect with a swollen, pale, anomalous, or normal optic disc seen on fundoscopy. Chiasmal disorders classically present with gradual onset of vision loss, bitemporal hemianopsia, and occasionally, endocrinopathy if the pituitary gland and/or hypothalamus are the causes or are involved. Advance in neuroimaging, especially magnetic resonance (MR) imaging, can reveal pathologic conditions previously detected only clinically. Some entities have imaging characteristics, leading to appropriate treatment without requiring tissue biopsies. Imaging also provides disease surveillance and posttreatment assessment, with computed tomography and MR imaging being complementary to each other.
Key points
- •
When clinical presentations of optic neuropathy and chiasm disorders are nonspecific, imaging plays a critical role in detecting, localizing, and differentiating the wide variety of causes.
- •
Some key imaging features are characteristic, leading to appropriate treatment and preventing unnecessary tissue biopsies.
- •
The following entities have characteristic imaging findings: optic neuritis, optic nerve sheath meningioma, optic nerve glioma, pituitary adenoma, aneurysm, etc.
Introduction
Optic neuropathy is characterized by loss of visual acuity, color vision (dyschromatopsia), and visual field defect. Fundoscopic examination may reveal a swollen, pale, anomalous, or normal optic disc. Chiasmal disorders classically present with gradual onset of vision loss, bitemporal hemianopsia, and occasionally, endocrinopathy if the pituitary gland and/or hypothalamus are the causes or are involved.
Introduction
Optic neuropathy is characterized by loss of visual acuity, color vision (dyschromatopsia), and visual field defect. Fundoscopic examination may reveal a swollen, pale, anomalous, or normal optic disc. Chiasmal disorders classically present with gradual onset of vision loss, bitemporal hemianopsia, and occasionally, endocrinopathy if the pituitary gland and/or hypothalamus are the causes or are involved.
Normal anatomy
Optic Nerve
Retinal ganglion cell axons form the optic nerves, which are myelinated by oligodendrocytes and supported by astrocytes. The optic nerve is divided into 4 segments: intraocular, intraorbital, intracanalicular, and intracranial ( Fig. 1 ). The optic nerve is isointense to cerebral white matter on T1-weighted and T2-weighted images. The optic nerve is surrounded by the optic nerve sheath, which contains pia, cerebrospinal fluid, arachnoid, and dura.
Optic Chiasm
The nasal retinal fibers of the optic nerve cross at the optic chiasm, whereas the temporal fibers remain uncrossed ( Fig. 2 ). The chiasm is surrounded by multiple critical structures including the pituitary gland inferiorly, infundibulum posteriorly, third ventricle and hypothalamus superiorly, inferior frontal lobe anteriorly, and supraclinoid internal carotid artery laterally. The chiasm ( Fig. 3 ) is typically located directly above the pituitary gland; in a minority of cases, it may be in prefixed (above the tuberculum sella) or postfixed positions (above the dorsum sella). Mass lesions of the pituitary gland typically compress the central portion of chiasm resulting in classic bitemporal hemianopsia, which may not present in cases of prefixed or postfixed chiasms.
Imaging protocol
Computed Tomography Versus MR Imaging
Computed tomography (CT) and MR imaging are complementary to each other in identifying anatomy and pathology of optic nerve and chiasm. MR imaging provides exquisite details of optic nerve and chiasm because of high tissue contrast differentiation. CT is valuable in the evaluation of bony structures (optic canals), calcified lesions, and metallic foreign bodies when MR imaging is contraindicated.
MR Imaging Protocol
Optic nerve and sella MR imaging protocols are suggested in Box 1 . Fat saturation sequences with intravenous gadolinium are helpful in detecting optic neuritis, neoplasms, postoperative changes, and optic nerve infarction.
Orbit
FOV: 18 cm
Matrix: 512 × 512
Thickness: 2 mm
Sequences
- •
Axial T1 TSE
- •
Axial T2 TSE/FS
- •
Coronal T2/FS
- •
Axial and coronal T1/FS/Gd
- •
Axial or coronal DWI
- •
Oblique sagittal T1/FS/Gd
- •
Thin-cut 3-dimensional heavily T2
- •
Axial T2 TSE brain
- •
Full brain MR imaging protocol with and without Gd (multiple-sclerosis-associated optic neuritis)
- •
Sella
FOV 15 cm
Sequences
- •
Sagittal T1 and T1/Gd
- •
Coronal T1 and T1/Gd
- •
Coronal T2
- •
Axial T2 brain
- •
Abbreviations: DWI, diffusion-weighted imaging; FOV, field of view; FS, fat saturation; Gd, gadolinium; TSE, turbo spin echo.
Pathology
Optic Neuropathy
Optic neuritis
Optic neuritis is a clinical diagnosis of optic nerve inflammation secondary to demyelination. A typical clinical scenario is a young white woman presenting with rapid onset of painful vision loss with optic disc swelling seen on fundoscopy. In cases with typical presentations, MR imaging does not aid in the diagnosis and does not alter the clinical course, management, or final visual outcome. If performed, MR imaging may show enlargement and enhancement of the nerve with hyperintensity on T2-weighted images in over 90% of cases ( Fig. 4 A–D). The findings are often similar to those of other inflammatory (eg, sarcoidosis) and infectious causes of optic neuropathy. However, involvement of the optic nerve, optic nerve sheath, and ciliary body favors the diagnosis of sarcoidosis over optic neuritis associated with multiple sclerosis (MS). Visual outcome is worse with longitudinally extensive lesions and with involvement of the intracanalicular segment (rigid bony canal). Eye pain correlates with intraorbital segment involvement, which is the most common segment to be involved. Enhancement suggests active disease, while resolution of enhancement is seen in the recovery phase.
Patients with optic neuritis are at increased risk for developing MS; it can occur as the first presentation of MS or develop later on. The presence of abnormal cerebral white matter lesions on MR imaging is the most important predictor for the development of MS in the future. It was found that 60% to 90% of patients with optic neuritis with abnormal cerebral white matter eventually develop MS. Only 20% to 25% of patients with normal-appearing MR images developed MS. If there are typical brain lesions (T2-hyperintense periventricular and juxtacortical white matter lesions, see Fig. 4 C), the administration of high-dose intravenous steroids has been found to decrease the incidence of MS development if given within 2 years. Therefore detection of brain lesions in patients with optic neuritis is a crucial task of radiologists.
Neuromyelitis optica (Devic disease)
Neuromyelitis optica (NMO) is a distinct severe autoimmune demyelinating disease mainly affecting the optic nerves and spinal cord. Acute optic neuritis in NMO is typically bilateral and severe (see Fig. 4 E–H). Longitudinally extensive lesions (≥3 vertebral segments), which may occupy the entire cross section of the cord, characterize spinal cord involvement. Brain lesions can be found in locations similar to those of MS as well as in atypical areas including hypothalamus, periaqueductal gray, and area postrema. Most patients test positive for aquaporin-4 antibody. Prognosis is poor, and treatment is different from that of MS.
Optic perineuritis
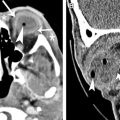
Optic perineuritis occurs when there is inflammation of the optic nerve sheath. The nerve itself is not involved. MR imaging shows enhancement and enlargement of optic nerve sheath, which may mimic a meningioma ( Fig. 5 ). Optic perineuritis can be idiopathic; however, it may be associated with some conditions such as inflammatory orbital syndrome, sarcoidosis, syphilis, Lyme disease, and tuberculosis. Coexisting intracranial leptomeningeal enhancement suggests an infection or neoplasm (leptomeningeal carcinomatosis [from breast or lung carcinomas] or lymphomatosis [from lymphoma]) as the cause.
Ischemic optic neuropathy
Acute interruption of the blood flow to the optic nerve can cause ischemic optic neuropathy (ION), typically presenting as acute painless vision loss in the elderly. ION is classified as anterior (intraocular segment) and posterior (retrobulbar segment) types. Anterior ischemic optic neuropathy (AION) is further classified as arteritic AION (A-AION) and nonarteritic AION (NA-AION). The distinction between A-AION and NA-AION is shown in Table 1 .
| Features | A-AION | NA-AION |
|---|---|---|
| Age (y) | >60 | >55 |
| Sex | Females > males | Females = males |
| Vision loss | Severe | Less severe |
| Risk factors/association | Giant cell arteritis (headache, scalp tenderness, jaw claudication) | Disc with small central cup, hypertension, hypercholesterolemia, diabetes mellitus, nocturnal systemic hypotension, obstructive sleep apnea, anemia, etc. |
| Fundoscopic finding | Pale edematous disc cotton wool spots | Hyperemic edematous disc |
| MR imagine | Restricted diffusion with enhancement | Restricted diffusion without enhancement |
| Treatment | Immediate corticosteroid | No specific treatment, risk factor modification, steroid is controversial |
Posterior ischemic optic neuropathy (PION) is relatively rare. The optic nerve head is normal on fundoscopy. MR imaging may show restricted diffusion of the retrobulbar optic nerve in the early phase ( Fig. 6 ) with atrophy seen after 6 to 12 weeks. The posterior segment of the optic nerve, especially the central portion, is more vulnerable to ischemia than the anterior segment because of less vascular supply (the anterior segment has dual arterial supplies from the central retinal and posterior ciliary arteries). PION can be seen in patients with giant cell arteritis, perioperative hypotension, vasculopathic risk factors, infection (varicella zoster, herpes), systemic inflammation (lupus, polyarteritis nodosa), and compressive optic neuropathy.
Optic Nerve and Optic Nerve Sheath Neoplasms
Optic nerve gliomas
Optic nerve gliomas (ONGs), as a part of the optic pathway gliomas (OPGs), are slowly growing low-grade astrocytomas, and not hamartomas. Most patients are children younger than 8 years. OPGs are one of the diagnostic criteria of neurofibromatosis type 1 (NF1) with approximately 20% of patients with NF1 having OPGs. NF1 is the most common syndrome associated with OPGs, therefore all new cases of OPGs should be screened for NF1. Syndromic and sporadic OPGs have different clinical/imaging features that may dictate different treatment and counseling, indicated in Table 2 . Locations of tumors (optic nerve, chiasm, or retrochiasm) do not show difference in visual outcome.
| Features | Syndromic | Sporadic |
|---|---|---|
| Age | Younger children | Older children |
| Location | Optic nerve with or without chiasm | Chiasm and retrochiasm |
| Morphology | Tubular, kinked | Fusiform |
| Bilaterality | More common | Less common |
| Behavior | Indolent, progress slower, asymptomatic, incidentally found during screening | More invasive, progress faster, symptomatic |
| Visual loss | Less common | More common |
| Proptosis | More pronounced | Less pronounced |
| Increased intracranial pressure and hydrocephalus | Less common | More common |
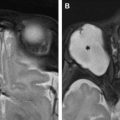
The roles of MR imaging are to differentiate ONGs from other intraconal tumors, evaluate orbital apex/intracranial extension, assess interval growth, and screen patients with NF1 with abnormal ophthalmic findings. Characteristic MR imaging findings can often preclude biopsies. On MR imaging, the tumors can be fusiform or kinked, showing T1 hypointensity, T2 isointensity to hyperintensity, and variable enhancement ( Fig. 7 ). The periphery of the tumor may appear T2 hyperintense, representing arachnoidal gliomatosis (leptomeningeal involvement). At times there will be significant arachnoid hyperplasia, resulting in discrepancy in size measurement on imaging and gross pathology (actual tumor size on pathology is smaller). Some ONGs may not enhance and look like cystic lesions.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree