Fig. 13.1
The Parks classification of perianal fistulas. a Intersphincteric fistula, b transsphincteric fistula, c suprasphincteric fistula, d extrasphincteric (translevator) fistula (Reprinted with permission from reference no. [27])
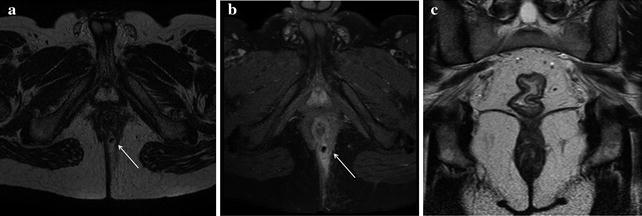
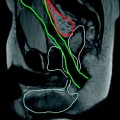
Fig. 13.2
Male patient with active UC and evidence of purulent perianal discharge. a On MRI axial T2-weighted sequences, a small simple transsphincteric hyperintense fistulous tract was detected at 5 o’clock. b T1-weighted fat saturated axial sequences after intravenous contrast administration showed vivid enhancement of the fistula and a better visualization of a tiny hypointense air bubble within the lumen. c In the same examination, T2-weighted coronal sequences imaged the rectum which presented thickened and hyperintense walls indicating active inflammation
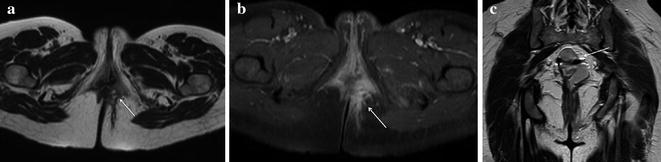
Fig. 13.3
Female patient with IPAA complaining of perianal pain. a On MRI axial T2-weighted sequences a complex transsphincteric hyperintense fistulous tract was detected at 3 o’clock. b T1-weighted fat saturated axial sequences after intravenous contrast administration showed vivid enhancement of the fistula and allowed to depict its small multiple ramifications. c In the same examination, T2-weighted coronal sequences offered an optimal view of the ileal pouch reservoir identified by hypointense ferromagnetic artifacts (signal voids), the anal canal and levator ani muscles, and the fistulous primary track in left ischioanal fossa (Reprinted with permission from reference no. [38])
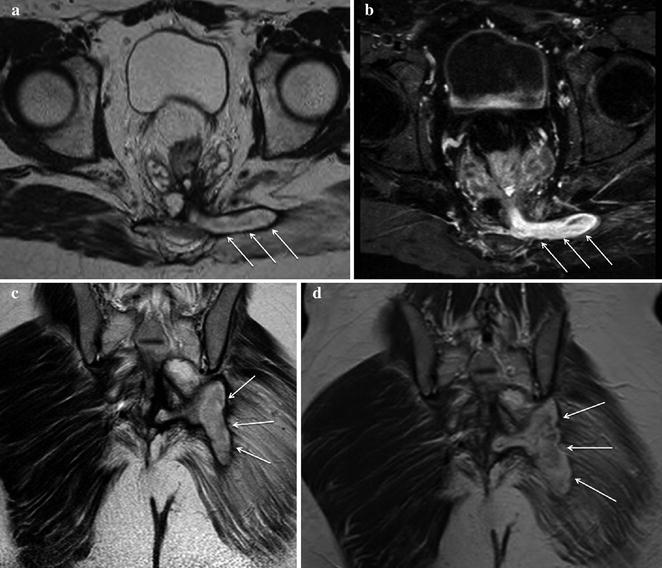
Fig. 13.4
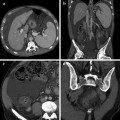
Male patient after proctocolectomy with IPAA complaining of perianal pain and massive purulent perianal discharge. a MRI T2-weighted axial sequences showed a large hyperintense fistula originating from the pouch-anal anastomosis level at 6 o’clock directed toward the left gluteal muscle. b On T1-weighted fat saturated axial sequences after intravenous contrast administration the large fistulous track strongly enhanced because of the presence of granulation tissue, confirming active inflammation. c T2-weighted coronal sequences best depicted the extent of the fistula in the posterior perineum and documented the involvement of left gluteal muscle which presented hyperintense signal suggestive of oedema. d On post-contrast T1-weighted coronal sequences the fistula showed diffuse enhancement
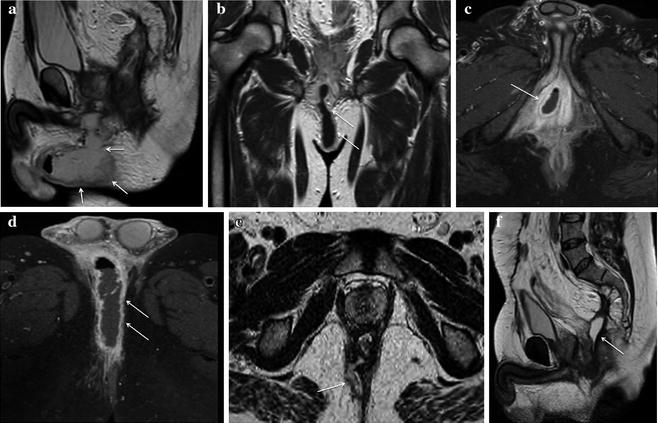
Fig. 13.5
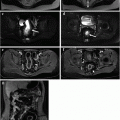
Male patient with IPAA, fever and strong perianal and genital pain. a On MRI T2-weighted sagittal sequences a bulky hyperintense fluid collection was detected in the anterior perineum, extending toward the scrotum. b T1-weighted coronal sequences after intravenous contrast administration best depicted a large fistulous track originating from the pouch-anal anastomosis level between 6 and 7 o’clock. Inflamed granulation tissue in the walls of the fistula presented bright signal, whereas fluid in the lumen remained T1-hypointense. c, d Post-contrast T1-weighted fat saturated axial sequences highlighted the presence of a large abscess in the right anterior perineum on the way of the transsphincteric fistula involving the crus of the penis and the scrotum. The collection showed typical enhancing walls, purulent hypointense content and a tiny fluid-air level. e, f MRI performed on the same patient after surgical drainage of the abscess. T2-weighted axial sequences (e) assessed a satisfactory outcome of the procedure: only a small fistolous track remained. In the same examination, T2-weighted sagittal sequences (f) better portrayed the complete surgical abscess drainage in the anterior perineum, but pointed out the occurrence of a hyperintense fluid collection posterior to the pouch-anal anastomotic site in the presacral space indicative of a sinus (Reprinted with permission from reference no. [38])
The St James’s University Hospital classification was proposed by radiologists on the basis of imaging findings and related the Parks surgical classification to anatomic MR imaging findings in the axial and coronal planes [28]. It uses anatomic landmarks in the axial plane familiar to radiologists and considers the primary fistulous track as well as secondary extensions and abscesses in evaluating and classifying fistulas. The classification grades fistulas into five groups: Grade 1, simple linear intersphincteric fistula; Grade 2, intersphincteric with abscess or secondary track; Grade 3, transsphincteric; Grade 4, transsphincteric with abscess or secondary track in ischiorectal or ischioanal fossa; Grade 5, supralevator and translevator [25].
The role of computed tomography (CT) in the evaluation of PAD is limited because CT, exposing patients to significant amounts of ionizing radiations, is not suitable for follow-up. Conversely, in emergency settings CT examinations are widely requested to investigate acute abdominal/perianal pain in UC patients, and to assess clinically suspected complications during the early post-operative period in patients undergoing IPAA. Since current fast multidetector scanners allow a panoramic coverage of the entire abdomen and pelvis in a few seconds and provide isotropic image reconstructions on arbitrary planes, longer coverage including the perianal region is suggested when examining acute UC patients. Intravenous iodinated contrast medium is usually injected unless contraindicated [23, 29].
Perianal abscesses and fistulas that are filled by air or fluid can be identified on CT because of adequate contrast between their hypodense contents and the enhancing, inflamed granulation tissue present in the walls (Fig. 13.6) [29]. CT is accurate in detecting perianal abscesses, and the involvement at the level of or above the levator ani muscles is identifiable on coronal images [29]. However, intrinsic CT contrast resolution limits the differentiation between different soft-tissue structures such as the pelvic muscles, active inflammatory changes and fibrotic scar tissue and CT does not have sufficient accuracy to correctly classify fistulas. Inhomogeneity, abnormal density or enhancement involving the ischioanal fat spaces indicate the presence of transsphincteric inflammation to be further investigated with MRI [29, 30].
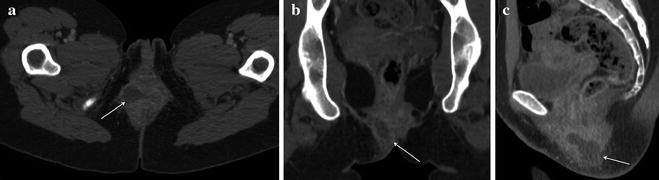

Fig. 13.6
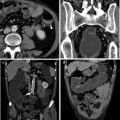
Female patient with active UC and fever, complaining of pain in perianal region. a In an emergency setting, CT detected a large perianal abscess with hypodense contents and enhancing walls in the intersphincteric space extending in the right ischioanal fossa. b Coronal reformations showed that the abscess did not cross the levator ani muscle and was limited to perianal space. c Sagittal reformations showed the real extent of the abscess and the involvement of the ipsilateral gluteus
CT is reliable in the diagnosis of pouch-related septic complications, suggested by the detection of extraluminal air, fluid or contrast material [23]. At CT, the ileal pouch reservoir is identified as a fluid-filled structure in the anatomical location of the resected rectum, with metallic surgical staples located 180° to each other. The pouch-anal anastomosis, indicated by the presence of hyperdense clips, should also be identified as it represents a possible source of leakage. In order to rule out early anastomotic leakage after IPAA surgery, bowel opacification with water-soluble contrast per os or per rectum may be useful [23].
Other imaging modalities currently used in the evaluation and follow-up of PAD are transanal and transperineal ultrasound which are easy to perform, low cost, ionizing radiation free and accurate in detecting perianal fistulas and abscesses with results comparable to pelvic MRI [30–34].
Transanal ultrasound, performed using a high-frequency rotating endoprobe, can accurately assess and classify perianal complications of UC, whether spontaneously occurring or complicating IPAA, providing at the same time a sonographic assessment of the rectal wall [31].
Perineal ultrasound, performed using high-frequency curved microconvex and linear-array probes, assesses fistulous and sinus tracts as well as abscesses and collections, documenting their relationship with the anal canal, the scrotum in men, and the labia and vagina in women [31]. In particular, perineal ultrasound is useful to detect perianal fistulas and inflammatory tissue or masses located outside the field of view of transanal ultrasound, or when the latter examination cannot be performed in a patient with severe anal pain or stricture [31].
Fistula tracts and abscesses complicating UC resemble those of Crohn’s disease. Fistulous tracts may be described as hypoechoic, round to oval structures in an intersphincteric location, sometimes with internal hyperechoic echoes as a result of gas or air within an abscess or a fistulous tract [31]. An opening within the mucosa of the anal canal or within the rectum may identified as a gap or disruption of the integrity of the echo structure of the normal wall [31]. An abscess may be detected sonographically as a mass which is either anechoic (without internal echoes) or hypoechoic (with internal echoes secondary to cellular debris) [30].
The limits of these techniques are the dependency on the expertise of the operators and on the availability of the necessary diagnostic tools.
13.4 Imaging of Genital Tract Involvement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree