Retrochiasmal visual pathways include optic tracts, lateral geniculate nuclei, optic radiations, and striate cortex (V1). Homonymous hemianopsia and field defect variants with relatively normal visual acuity suggest that the lesions involve retrochiasmal pathways. From V1, visual input is projected to higher visual association areas that are responsible for perception of objects, faces, colors, and orientation. Visual association areas are classified into ventral and dorsal pathways. Damage to the ventral stream results in visual object agnosia, prosopagnosia, and achromatopsia. Balint syndrome, visual inattention, and pure alexia are examples of dorsal stream disorders. Posterior cortical atrophy can involve ventral and dorsal streams, often preceding dementia.
Key points
- •
Retrochiasmal visual pathways include optic tracts, lateral geniculate nuclei, optic radiations, and striate cortex.
- •
Homonymous hemianopsia and field defect variants with relatively normal visual acuity suggest that the lesions are in retrochiasmatic areas.
- •
Higher visual association areas (VAA) interpret visual information from the primary visual cortex, resulting in perception of objects, faces, colors, and orientation.
- •
Each VAA has a specific function.
- •
Lesions involving VAA produce impairment of the specific abnormal visual perception despite normal visual fields.
Introduction
The retrochiasmal (or retrochiasmatic) visual pathways consist of the optic tracts, lateral geniculate nuclei (LGN), optic radiations (ORs), and striate cortex (primary visual cortex). Diseases involving the retrochiasmal visual pathway typically result in contralateral homonymous hemianopsia with relatively intact visual acuity. The primary visual cortex (V1) projects information to the higher visual cortex or visual association areas (V2, V3, and so forth), where more complex visual perceptions are processed. Patients suffering from higher cortical dysfunction may have specific visual perception abnormalities including achromatopsia, prosopagnosia, and Balint syndrome, despite normal visual acuity and fields. This article elucidates the anatomy and pathology of diseases affecting these particular visual pathways, focusing on imaging and clinical correlation.
Introduction
The retrochiasmal (or retrochiasmatic) visual pathways consist of the optic tracts, lateral geniculate nuclei (LGN), optic radiations (ORs), and striate cortex (primary visual cortex). Diseases involving the retrochiasmal visual pathway typically result in contralateral homonymous hemianopsia with relatively intact visual acuity. The primary visual cortex (V1) projects information to the higher visual cortex or visual association areas (V2, V3, and so forth), where more complex visual perceptions are processed. Patients suffering from higher cortical dysfunction may have specific visual perception abnormalities including achromatopsia, prosopagnosia, and Balint syndrome, despite normal visual acuity and fields. This article elucidates the anatomy and pathology of diseases affecting these particular visual pathways, focusing on imaging and clinical correlation.
Normal anatomy
Optic Tract
Each optic tract is composed of ipsilateral temporal fibers and contralateral nasal fibers; therefore, it corresponds to the contralateral visual hemi-field (ie, the left optic tract corresponds to the right visual field and vice versa). The optic tract projects posterolaterally. It is ventral to the rostal midbrain, globus pallidus, and thalamus; anterior to the cerebral peduncle; and medial to the uncus ( Fig. 1 ). Most of the fibers terminate in the LGN with some synapsing at the pretectal nuclei, which is responsible for the pupillary light reflex.
Lateral Geniculate Nucleus
LGN is one of the thalamic nuclei and contains six neuronal layers that receive visual input from the optic tracts. Layers 2, 3, and 5 receive input from the ipsilateral eye, and layers 1, 4, and 6 receive input from the contralateral eye. Visual information is projected to the striate cortex via OR. The LGN has characteristic anatomy on MR imaging, best visualized on coronal thin-cut three-dimensional T1-weighted images, showing it located superior to the ambient cistern and superomedial to the hippocampal body ( Fig. 2 ).
Optic Radiations
ORs (see Fig. 4 of Advanced MR Imaging of the Visual Pathway) are white matter tracts connecting LGN and striate cortex. Each OR contains three bundles: (1) dorsal, (2) central, and (3) anterior (Meyer loop). The dorsal bundle is located in the parietal lobe, carrying information from the lower visual field, and terminates in the striate cortex superior to the calcarine sulcus. The central bundle and Meyer loop are found in the temporal lobe, carrying information from the superior visual field to the striate cortex, inferior to the calcarine sulcus. Meyer loop is prone to damage from anterior temporal lobectomies because of its course along the anterior aspect of the temporal horn.
Striate Cortex
The striate cortex is located in the medial aspect of the occipital lobe ( Fig. 3 ), serving as the primary visual area (Brodmann area 17 or V1). It demonstrates retinotopic organization, with each retinal ganglion cell having a specific projection on striate cortex, preserving point-to-point mapping. The occipital tip corresponds to central (macular) vision, whereas the more anterior regions correspond to peripheral vision. The peripheral visual areas are supplied by the posterior cerebral arteries (PCA), whereas the central visual area (occipital tip) has a dual arterial supply from the PCA and middle cerebral arteries (MCA).
Visual Association Areas
Area V1 receives basic visual information from the retina via geniculostriate pathways. Complex visual analysis occurs in visual association areas, which initially receive input from area V1. Areas V2 (Brodmann area 18) and V3 (Brodmann area 19) are situated immediately anterior to V1. Higher visual cortical processing is complex with extensive connections, and can be simplified as ventral occipitotemporal and dorsal occipitoparietal pathways ( Fig. 4 ).
The ventral pathway is located in the inferior aspect of the occipitotemporal lobes and contains area V4 (fusiform and lingual gyri). The ventral stream through area V4 represents color, shape, pattern, facial, and object recognition (“what” pathway). The dorsal stream is located in the lateral surface of the occipitotemporal lobes (area V5) and parietal lobes, and is responsible for spatial orientation, motion, and visual attention (“where” pathway). Another important aspect of higher visual analysis is the ability to precisely perceive multiple stimuli presented in sequential orders, to plan, and to react to potential threats, a so-called “when” pathway. Recently, researchers have found areas in the right parietal lobe, temporoparietal junction, and area V5 to be responsible for this crucial temporal discrimination role.
Pathology
Optic Tract
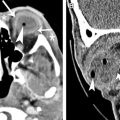
Isolated diseases of the optic tract are rare, and most lesions are contiguous with the optic chiasm including chiasmal neuritis, sarcoidosis, and glioma. Optic tract infarction is unusual because optic tract receives dual arterial supply from the anterior choroidal and posterior communicating arteries. Other neoplasms of optic tracts have been reported, including astrocytomas ( Fig 5 ) and gangliogliomas. Craniopharygiomas and dolichoectatic basilar artery may compress the optic tracts.
Optic Tract Localization and Deep Brain Stimulation
Deep brain stimulation and pallidotomy are surgical treatment options for Parkinson disease. The target of deep brain stimulation and pallidotomy is the globus pallidus interna, which is situated immediately superior to the optic tracts. The use of MR imaging and neurophysiologic monitoring for stereotactic guidance allows for precise localization of the target and prevents damage to the optic tract.
Lateral Geniculate Nucleus
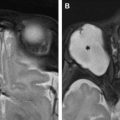
A lesion involving only the LGN is very uncommon. However, a spectrum of diseases may involve LGN and produces contralateral homonymous hemianopsia with characteristic sectoranopia. These include infarction ( Fig. 6 ), astrocytomas ( Fig. 7 ), metastasis, osmotic myelinolysis, and arteriovenous malformation.
Optic Radiation
The most common pathology affecting OR is stroke of MCA distribution, followed by hemorrhage, neoplasm ( Fig. 8 ), herpes simplex encephalitis, and postsurgical injury. Lesions involving the parietal and temporal lobe ORs may result in inferior and superior quadrantanopia, respectively. Anterior temporal lobectomy is an effective surgery for temporal lobe epilepsy; however, damage of Meyer loop may lead to a “pie-in-the-sky” defect, potentially precluding 4% to 50% of patients from driving ( Fig. 9 ).