This article briefly discusses the imaging approach to lesions of the anterior skull base. A brief review of normal anatomy and imaging techniques is followed by a discussion about common neoplastic and non-neoplastic pathologies involving this region.
Advances in neuroimaging over last 2 decades have contributed significantly to the development of skull-base surgery, including anterior skull-base (ASB) surgery. With precise preoperative imaging information as a roadmap, skull-base surgeons are reaching into places that were earlier considered unapproachable. Imaging helps in identifying features characteristic of certain pathology, in staging neoplasms preoperatively, in defining extent of tumor spread, in planning a surgical approach, and lastly in evaluating for tumor recurrences. Imaging plays a central role as lesions in this location are difficult to access for clinical inspection. Radiologists interpreting imaging studies in patients with ASB lesions need to be familiar with the intricate anatomy of this region and also need to use correct imaging protocols. CT scan and MR imaging are the primary modalities used to evaluate the ASB and these complement each other. Conventional angiogram, a useful adjunct in the workup of vascular lesions, has a role in preoperative embolization of such vascular tumors as juvenile nasopharyngeal angiofibroma. This article briefly discusses the imaging approach to lesions of the ASB. A brief review of normal anatomy and imaging techniques is followed by a discussion about common neoplastic and non-neoplastic pathologies involving this region.
Relevant anatomy
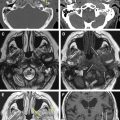
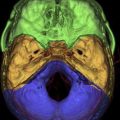
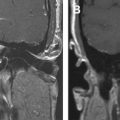
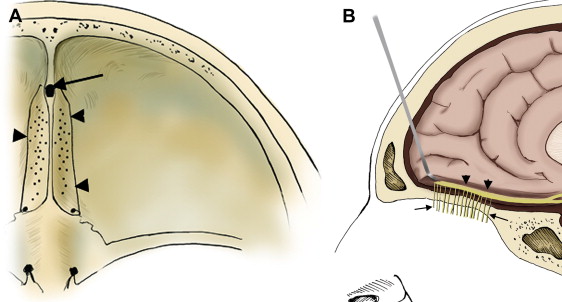
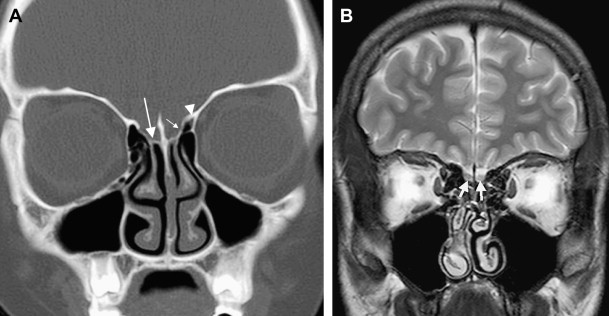
The ASB forms the bottom of the anterior skull, separating the anterior cranial fossa superiorly from the paranasal sinuses and the orbits below. It consists of the floor of the anterior cranial fossa and the frontal sinus, including the roof of the ethmoid sinuses, the nasal cavity, and the orbits. It extends from the posterior wall of the frontal sinus anteriorly to the posterior border formed by the lesser wing of the sphenoid bone ( Fig. 1 A ). Medially the floor of the anterior cranial fossa is formed by the roof of the nasal cavity and ethmoid sinuses. The thin cribriform plate of the ethmoid bone forms the roof of the nasal cavity, which has multiple small perforations transmitting olfactory nerves from the nasal mucosa to the olfactory bulb ( Figs. 1 B and 2 A ). The orbital plates of the frontal bone (orbital roof) are on either side laterally. The roof of the orbit, formed by the orbital plate of the frontal bone, constitutes the largest surface area of the anterior cranial fossa ( Fig. 2 ). The site where the anterior ethmoid artery enters the anterior cranial fossa (lateral lamella of the cribriform plate) is the site of common bony injuries and cerebrospinal fluid (CSF) leaks. The orbital plate of the frontal bone, unlike the cribriform plate, is thick and sturdy, and hence relatively resistant to erosion.
Imaging techniques
CT and MR imaging have complementary roles in the evaluation of ASB pathologies and are used together. CT is an excellent modality for delineation of fine bony details, whereas MR imaging provides superior soft tissue details and multiplanar information. CT has a good specificity in the diagnosis of fibro-osseous and primary bone lesions and in distinguishing an aggressive from a benign pattern of bone involvement. At our institute, the University of Michigan Health System, we obtain CT scans in at least two different planes with slices ranging in thickness from 1.25 to 2.5 mm. With the widespread use of multidetector CT scanners, a single volume acquisition in an axial plane is obtained (with 0.625- to 1.25-mm slice thickness) and multiplanar reconstructions in sagittal and coronal planes. We typically use intravenous contrast for evaluation of ASB mass lesions, but CT without contrast may be performed if the patient is having an MR image simultaneously and in patients where CT is used primarily to look for bony abnormalities.
MR imaging is more sensitive then CT for detecting soft tissue abnormalities. MR imaging is extremely helpful for evaluation of dural, leptomeningeal, and brain parenchymal involvement. MR imaging is also good for depicting early bone marrow invasion (where CT does not show cortical erosions) and for discriminating between retained secretions in paranasal sinuses from tumor. At our institution, we obtain conventional or fast spin echo T1-weighted images in axial and coronal planes, axial and coronal T2-weighted images, and post–contrast-enhanced images (with and without fat suppression) in all patients with suspected skull-base lesions. The images are obtained with higher resolution, small field of view with slice thickness of not more than 3 mm. Intravenous gadolinium is important to clearly delineate the tumor extent and to detect intracranial extension, particularly meningeal involvement. Fat-suppressed T1-weighted images can be obtained if the lesion is in the vicinity of fat-containing areas, such as the orbits. However, the skull-base region is extremely susceptible to artifacts from tissue inhomogeneity and fat-suppression technique is not always successful. To reduce the acquisition time of these high-resolution images, parallel imaging techniques (if available) can be helpful.
Imaging techniques
CT and MR imaging have complementary roles in the evaluation of ASB pathologies and are used together. CT is an excellent modality for delineation of fine bony details, whereas MR imaging provides superior soft tissue details and multiplanar information. CT has a good specificity in the diagnosis of fibro-osseous and primary bone lesions and in distinguishing an aggressive from a benign pattern of bone involvement. At our institute, the University of Michigan Health System, we obtain CT scans in at least two different planes with slices ranging in thickness from 1.25 to 2.5 mm. With the widespread use of multidetector CT scanners, a single volume acquisition in an axial plane is obtained (with 0.625- to 1.25-mm slice thickness) and multiplanar reconstructions in sagittal and coronal planes. We typically use intravenous contrast for evaluation of ASB mass lesions, but CT without contrast may be performed if the patient is having an MR image simultaneously and in patients where CT is used primarily to look for bony abnormalities.
MR imaging is more sensitive then CT for detecting soft tissue abnormalities. MR imaging is extremely helpful for evaluation of dural, leptomeningeal, and brain parenchymal involvement. MR imaging is also good for depicting early bone marrow invasion (where CT does not show cortical erosions) and for discriminating between retained secretions in paranasal sinuses from tumor. At our institution, we obtain conventional or fast spin echo T1-weighted images in axial and coronal planes, axial and coronal T2-weighted images, and post–contrast-enhanced images (with and without fat suppression) in all patients with suspected skull-base lesions. The images are obtained with higher resolution, small field of view with slice thickness of not more than 3 mm. Intravenous gadolinium is important to clearly delineate the tumor extent and to detect intracranial extension, particularly meningeal involvement. Fat-suppressed T1-weighted images can be obtained if the lesion is in the vicinity of fat-containing areas, such as the orbits. However, the skull-base region is extremely susceptible to artifacts from tissue inhomogeneity and fat-suppression technique is not always successful. To reduce the acquisition time of these high-resolution images, parallel imaging techniques (if available) can be helpful.
Neoplasms
Malignant Neoplasms—General Principles of Imaging
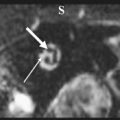
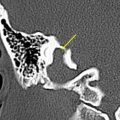
A large number of malignant and aggressive tumors involve the ASB and sinonasal cavities. Radiologists should try to refrain from giving the specific tissue diagnosis, as many of these lesions show similar features. However, it is extremely important to provide information about the lesion resectability, involvement of the bones, invasion of the meninges and brain parenchyma, and extension into the orbit and into the neurovascular structures, including the cavernous sinuses. This information should help the surgeon decide if the tumor is resectable and the best surgical approach for the same. As stated earlier, both CT and MR imaging should be used together for complete evaluation of malignant ASB lesions. Dural and intraparenchymal invasion increases the postoperative morbidity with decreased overall 5-year survival rate. Dural invasion by malignant tumors usually is seen as nodular thickening of the dura or dural thickness of more than 5 mm. Brain parenchymal involvement is seen as varying degrees of vasogenic edema with or without contrast enhancement ( Fig. 3 A and B ). Orbital invasion occurs most frequently through lamina papyracea, the weakest of all orbital walls ( Fig. 3 C). After breaching the bone, tumor extension is still limited by fibrous periorbita. The strong periorbital is attached along the superior and inferior aspect of the medial wall and can be elevated but remains attached at these areas. Even if the periorbital limits the tumor in the posterior orbit, tumor can break through anteriorly. Tumors transgressing the periorbita and those extending into the orbital apex require orbital exenteration with sacrifice of the optic nerve.
Esthesioneuroblastoma
Esthesioneuroblastomas, also known as olfactory neuroblastomas, comprise 3% of intracranial tumors and arise from the specialized olfactory epithelium. Because of their origin, they are similar to other tumors arising from the neural crest, such as primitive neuroectodermal tumors, small-cell lung carcinomas, and neuroblastomas. They have a bimodal distribution, primarily affecting patients in the second and fifth decades of life, and are slightly more common in men. Clinical presentation includes nasal obstruction, epistaxis, anosmia, pain, and mass effect. On CT scans, these lesions appear as solid, homogeneously enhancing superior nasal cavity masses with or without destruction of the adjacent bones, especially of the cribriform plate. Calcifications may occur in the tumor mass. Like other small-cell tumors, these tumors are slightly hyperdense on CT and of intermediate signal intensity on both T1- and T2-weighted images. In some of the larger tumors in which there is intracranial extension ( Fig. 4 ), peripheral tumor cysts can occur at the margins of the intracranial mass and, when present, are suggestive of the diagnosis. Meningeal spread and parenchymal invasion of the olfactory bulbs and inferior frontal lobes are often seen in advanced tumors.
Squamous cell cancer
Squamous cell carcinomas are the most common malignancies of the nose and paranasal sinuses. They have a male predominance, and average age at presentation is 50 years or more. The maxillary antrum is the most frequent primary site, followed by the nasal cavity, ethmoid sinus, and, rarely, frontal or sphenoid sinus. On imaging, these tumors are aggressive, often demonstrating bony destruction on CT scan. They show intermediate signal intensity on T2-weighted images, reflecting high cellularity ( Fig. 5 A ). They show intense homogeneous enhancement on postcontrast imaging ( Fig. 5 B), which helps to distinguish this entity from benign inflammatory processes, such as sinonasal polyposis, and from mucoceles, which do not erode bone and enhance peripherally. Because of the aggressive nature of squamous cell carcinomas, extension through the cribriform plate into the anterior cranial fossa is possible. Because of the presence of necrosis and hemorrhage, large tumors may be quite heterogeneous.
Adenocarcinoma
Adenocarcinomas comprise 10% of all sinonasal tumors and are subdivided into minor salivary gland tumors, intestinal-type adenocarcinoma, and neuroendocrine neoplasms. Sinonasal intestinal-type adenocarcinoma was so named because of its histologic resemblance to various intestinal tumors ranging from benign polyps to adenocarcinoma. These tumors are seen often in males from 55 to 60 years old and are more common in workers in hardwood and shoe industries. Imaging features are usually nonspecific and it is not possible to distinguish these tumors from squamous cell cancers based on imaging alone ( Fig. 6 ). Low-grade tumors show a less aggressive pattern of bone involvement, usually with remodeling and thinning rather than permeative destruction.
Adenoid cystic tumor
Arising in sinonasal minor salivary gland tissue, adenoid cystic carcinomas are slow but steady-growing lesions. Symptoms usually last an average of 5 years and are related to mass effect and neural involvement of tumor. These tumors are destructive and have a high propensity for perineural spread ( Fig. 7 A ). Trigeminal nerve involvement is most common in nasal/paranasal sinus tumors ( Fig. 7 B). Regional lymphatic spread is rare, while distant metastasis is more common, averaging 40%. CT scanning may show bony destruction, and postcontrast MR imaging is of particular use in identifying perineural spread of malignancy.