Pathologic processes within the cerebellopontine angle (CPA) may present because of mass effect on local structures or on the pons or cerebellum. MR imaging is superior to CT for evaluation of the complete extent of a CPA mass and for characterization of its likely nature. MR is also superior to CT for the detection of leptomeningeal processes. Arriving at a short list of differential diagnoses requires a methodical approach, keeping in mind the location, signal, diffusion, and enhancement characteristics of the lesions. Advanced MR imaging techniques, such as magnetic resonance spectroscopy, are not usually needed routinely but may aid in the differential diagnosis when presented with atypical masses.
There is a wide range of pathologic processes within the cerebellopontine angle (CPA). These processes may present because of mass effect on local structures, such as the fifth to 12th cranial nerves, or because of mass effect on the pons or cerebellum, which may result in fourth ventricular obstruction. MR imaging is vastly superior to CT for the evaluation of the complete extent of a CPA mass and for best characterization of its likely nature. MR imaging is also superior to CT for the detection of leptomeningeal processes. The authors’ approach to differential diagnosis of CPA lesions starts by grossly dividing pathologic findings into masses, vascular lesions, and more subtle leptomeningeal disease. Although the most common CPA mass is the benign vestibular schwannoma, there are many different masses and disease processes that may need to be considered in the complete differential diagnosis. The authors present their approach to CPA imaging with the critical anatomy, imaging strategies, and a differential diagnosis algorithm.
Cerebellopontine angle critical anatomy
The CPA, by definition, is the cisternal space anterior to the cerebellar hemispheres and lateral to the pons. It can also be thought of as an inverted triangular-shaped cerebrospinal fluid (CSF) space at the lateral aspect of the posterior fossa, with the base of the triangle formed by the tentorium. The lateral margin of this triangle is formed by the posterior aspect of the petrous temporal bone. The cistern extends into the petrous bone as the internal auditory canal (IAC), with its medial aperture being the porus acousticus. The fifth cranial nerve traverses the superior aspect of the cistern from the lateral pons to Meckel’s cave. The seventh (facial) and eighth (vestibulocochlear) cranial nerves arise from the low CPA cistern at the lateral aspect of the inferior medulla, emerging at the level of the foramen of Luschka. These nerves ascend to the porus acousticus, with the facial nerve slightly anterior and superior to the eighth cranial nerve. Both nerves are intimately related to the anterior inferior cerebellar artery (AICA), and in two thirds of patients, the AICA loops into the IAC on at least one side. The ninth through 11th cranial nerves arise from the medulla and upper cervical cord and traverse the inferior lateral aspect of the CPA cistern to exit the skull base at the jugular foramen. The 12th cranial nerve exits anteriorly through the hypoglossal canal of the basiocciput.
Imaging strategies
MR imaging and CT are complementary modalities, although MR imaging offers greater utility for the detection and characterization of CPA processes. The authors favor the use of heavily T2-weighted three-dimensional sequences, such as fast imaging using steady-state acquisition or constructive interference in the steady state, which provide high-resolution detail of cranial nerves and the blood vessels associated with them. Such thin-slice heavily T2-weighted imaging also allows good evaluation of the inner ear structures, which is important in the complete evaluation of sensorineural hearing loss. The authors always administer gadolinium and perform pre- and postcontrast 2-mm T1-weighted sequences in the axial and coronal planes. Fat saturation after contrast is always applied ( Table 1 ). CT is largely reserved for defining any bone changes that may be associated with CPA masses, such as meningiomas, but is not a first-line tool for evaluating or characterizing CPA masses.
| Sequence/Plane | Repetition Time/Echo Time | Field of View | Slice/Skip | No. Excitations |
|---|---|---|---|---|
| T1-weighted axial | 500/MF | 17 | 2 mm/0 | 2 |
| Three-dimensional, fast spin echo, T2-weighted | 3000/MF | 16 | 1 mm/0 | 1 |
| T1-weighted axial, after administration of gadolinium, fat saturated | 500/MF(17) | 17 | 2 mm/0 | 2 |
| T1-weighted coronal, after administration of gadolinium, fat saturated | 500/MF | 17 | 2 mm/0 | 2 |
Imaging strategies
MR imaging and CT are complementary modalities, although MR imaging offers greater utility for the detection and characterization of CPA processes. The authors favor the use of heavily T2-weighted three-dimensional sequences, such as fast imaging using steady-state acquisition or constructive interference in the steady state, which provide high-resolution detail of cranial nerves and the blood vessels associated with them. Such thin-slice heavily T2-weighted imaging also allows good evaluation of the inner ear structures, which is important in the complete evaluation of sensorineural hearing loss. The authors always administer gadolinium and perform pre- and postcontrast 2-mm T1-weighted sequences in the axial and coronal planes. Fat saturation after contrast is always applied ( Table 1 ). CT is largely reserved for defining any bone changes that may be associated with CPA masses, such as meningiomas, but is not a first-line tool for evaluating or characterizing CPA masses.
| Sequence/Plane | Repetition Time/Echo Time | Field of View | Slice/Skip | No. Excitations |
|---|---|---|---|---|
| T1-weighted axial | 500/MF | 17 | 2 mm/0 | 2 |
| Three-dimensional, fast spin echo, T2-weighted | 3000/MF | 16 | 1 mm/0 | 1 |
| T1-weighted axial, after administration of gadolinium, fat saturated | 500/MF(17) | 17 | 2 mm/0 | 2 |
| T1-weighted coronal, after administration of gadolinium, fat saturated | 500/MF | 17 | 2 mm/0 | 2 |
Approach to differential diagnosis
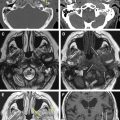
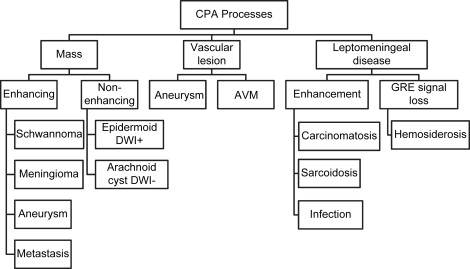
The authors’ approach to the differential diagnosis of CPA lesions starts by grossly dividing pathologic changes into masses, vascular lesions, and more subtle leptomeningeal disease ( Fig. 1 ). Vestibular schwannoma is the most common CPA mass, with meningioma, epidermoid, arachnoid cyst, metastasis, and aneurysm accounting for the remainder of most masses. Masses can be further subdivided into those that enhance, such as schwannoma, meningioma, aneurysm, and metastases, and nonenhancing lesions, such as arachnoid cyst and epidermoid. The latter two masses are readily distinguished by the presence of restricted diffusion in an epidermoid as compared with increased diffusion in an arachnoid cyst. An aneurysm most commonly arises from the AICA and is the most common vascular lesion of the CPA. It may also be found in association with an arteriovenous malformation (AVM). Aneurysms and AVMs are well evaluated with CT angiography or magnetic resonance angiography, although complete study may require catheter angiography. Many leptomeningeal processes present as cranial neuropathies, and most are evident because of the presence of enhancement. Leptomeningeal processes are typically much more subtle imaging findings, even with MR imaging. The 3-mm thin-slice imaging allows better detection of subtle abnormal enhancement as compared with routine 5- to 7-mm thickness whole-brain imaging. The presence of leptomeningeal enhancement alone results in a wide differential diagnosis, which may require CSF sampling for definitive differentiation of processes, such as sarcoidosis, carcinomatosis, and infections. Hemosiderosis does not enhance but is evident by T2 ∗ signal loss of the leptomeninges.
Cerebellopontine angle masses
Vestibular Schwannomas
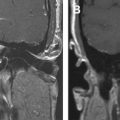
Vestibular schwannomas account for 70% to 80% of all CPA lesions. Previously known as acoustic neuromas, these lesions most often arise from the inferior vestibular nerve within the IAC and present with hearing loss or tinnitus. Schwannomas can be entirely intracanalicular or have intracanalicular and cisternal components, resulting in the description of an “ice-cream cone” tumor ( Fig. 2 ). Rarely, vestibular schwannomas are purely intracisternal. Intracisternal tumors tend to reach a larger size before presenting with mass effect on the cerebellar hemispheres and fourth ventricle rather than hearing loss. CPA masses, such as cisternal schwannomas, may cause mass effect on the fifth cranial nerve also.
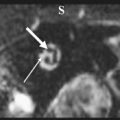
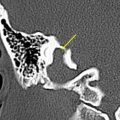
T2-weighted and, in particular, postcontrast imaging can reveal subtle imaging variations in vestibular schwannomas. Although small lesions tend to have homogeneous enhancement, larger lesions more frequently have cystic spaces and demonstrate heterogeneous enhancement. Those tumors with cystic areas tend to be faster growing and to have a less favorable surgical outcome with regard to integrity of facial nerve function. These heterogeneous lesions may also contain more hemosiderin deposition, which can alter the signal intensity further. Rarely, acute hemorrhagic expansion or degeneration of a schwannoma with enlargement can cause a patient to present with sudden onset of vertigo or emesis ( Figs. 3 and 4 ). Treatment options for vestibular schwannomas include observation, microsurgical removal, and stereotactic radiosurgery. Observation is reserved for elderly patients who do not experience mass effect. Surgical outcome has improved with the newer microsurgical techniques and instrumentation, including intraoperative facial nerve monitoring and brainstem evoked potential recording.
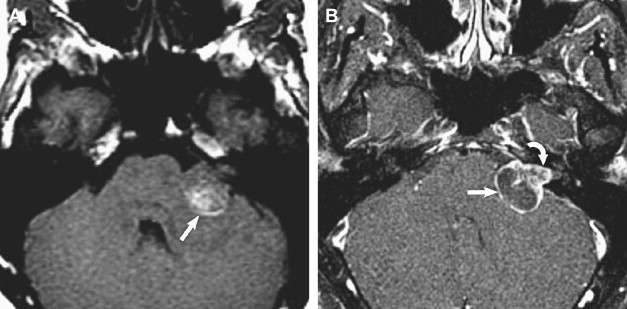
Treatment options depend, to a large extent, on tumor size, tumor extent into the IAC, and presence of cystic components. Hence, when describing these masses, it is important to describe the tumor dimensions in terms of the cisternal component and the component within the IAC. Lateral extent of the tumor within the IAC should be assessed, and particular mention should be made of whether or not the mass extends up to or into the cochlear aperture. If the mass is associated with a cyst, the size of this cyst should be described.
Surgical approaches include the subtemporal middle cranial fossa approach, retrosigmoid approach, and translabyrinthine approach. With the greater use of radiosurgery (“gamma knife”), the middle fossa approach is used less often in the authors’ institution because this is largely reserved for smaller predominantly IAC masses. The retrosigmoid approach is preferred for those schwannomas with a large CPA component because it allows good access to the entire CPA. The translabyrinthine approach results in complete hearing loss and is generally reserved for those tumors that extend far laterally within the IAC, because it is difficult to reach these lesions from a retrosigmoid approach. A combination of the retrosigmoid and translabyrinthine approach is used for large complicated lesions involving the IAC and CPA. Immediate postoperative complications depend on the surgical approach used and include contusions of the cerebellum or AICA ischemia. Dural venous sinus thrombosis is also a well-described but uncommon complication. When evaluating postoperative scans after removal of vestibular schwannomas, dural linear enhancement is expected in the IAC and along the CPA margins. Attention should be paid to developing nodular enhancement, which might suggest recurrent growth ( Fig. 5 ).
Radiosurgical techniques include single-session gamma knife radiosurgery (GKS) or linear accelerator or fractionated therapy, such as Cyberknife therapy. Of these, the best-studied and most widely used technique is GKS. GKS has emerged as the treatment of choice in many centers because of the potential advantages it offers in terms of faster recovery, reduced cost, and minimized morbidity and mortality. GKS is generally used for tumors when the cisternal component is less than 3 cm. In the first 6 to 12 months after treatment, tumors may depict more heterogeneous enhancement with areas of central necrosis. Overall tumor size may actually increase for the first 2 years, which is why tumors larger than 3 cm are excluded from this treatment modality. After this time, gradual regression in size can be expected. Hydrocephalus from mass effect attributable to increased tumor size can be treated with shunting or steroids. Development of cranial neuropathies warrants surgical intervention.
Meningiomas
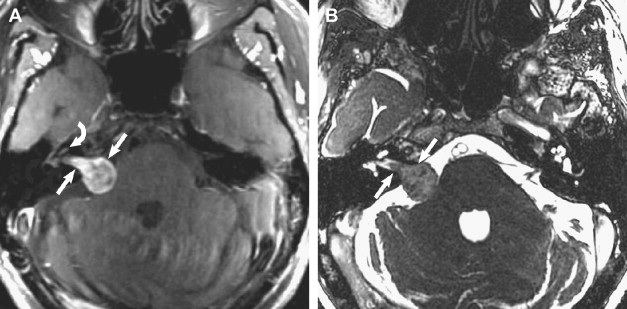
Meningiomas are the second most common tumor to arise in the CPA, representing up to 10% to 15% of tumors. These slow-growing masses arise from arachnoid meningoepithelial cells and, as with meningiomas elsewhere, occur more frequently in individuals older than the age of 40 years. In the CPA, these tumors tend to arise from the dura of the dorsal aspect of the petrous temporal bone. They typically present with hearing loss, tinnitus, and headache, whereas larger tumors may present with cerebellar signs and trigeminal neuropathy. On MR imaging, the masses are isointense to cerebral cortex on T1- and T2-weighted sequences and depict avid postcontrast enhancement. Because meningiomas may involve the porus acousticus and extend into the IAC, the detection of a dural tail is a key finding to differentiate meningiomas from vestibular schwannomas ( Figs. 6 and 7 ). Other imaging features to facilitate this imaging differentiation are identified more readily on CT: the presence of tumoral calcifications and hyperostosis of adjacent bone. Advanced MR imaging techniques have attempted to distinguish these tumors further. Although subtle differences have been reported using diffusion-weighted imaging (DWI), perfusion-weighted imaging, and magnetic resonance spectroscopy (MRS), there is considerable overlap of findings.