Preterm birth is birth before 37 weeks’ gestational age. The incidence of preterm birth is approximately 1 in 10 births in the United States. The imaging of a preterm infant is not uncommon, and especially in the neonatal period, the radiologist plays a crucial role in the diagnosis of common complications related to preterm birth. Through a system-based approach, this chapter will review key imaging diagnoses commonly seen in the preterm infant.
Is This Neonate Preterm?
When evaluating any neonatal radiograph, a helpful initial step is to determine whether the neonate is preterm. Signs of prematurity include overall reduction in subcutaneous fat and lack of humeral head ossification. Humeral head ossification is almost never present before 38 weeks’ gestational age.
Lines and Tubes in the Neonatal Intensive Care Unit
Umbilical Venous Catheter
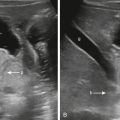
Umbilical venous catheters (UVCs) are used in critically ill neonates for venous access for administration of intravenous fluids, parenteral nutrition, blood products, and medical medications, because peripheral and conventional central venous catheters are difficult to place. The typical course of the UVC is from the umbilical vein superiorly into the left portal vein, after which it courses through the ductus venosus into the inferior vena cava. The ideal positioning of the UVC is at the inferior vena cava–right atrial junction ( Fig. 18.1 ).

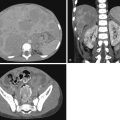
Because of lack of imaging guidance during UVC placement, misplacement is not uncommon ( Fig. 18.2 ). Complications of UVC misplacement include hepatic hematoma and necrosis in the setting of an intrahepatic placement of a UVC ( Fig. 18.3 ), right or left atrial perforation, or umbilical vein perforation resulting in extravasation or hemoperitoneum ( Fig. 18.4 ). Thrombosis in the inferior vena cava may occur even in an appropriately positioned UVC.



Umbilical Arterial Catheter
Umbilical arterial catheters (UACs) are used in neonates for blood pressure monitoring, blood sampling, and infusion of fluids and medications. The course of the UAC is from one of the two umbilical arteries inferiorly into the right or left internal iliac artery, after which it ascends through the common iliac artery into the aorta. A UAC may be positioned with the tip between T6 and T9 (high UAC) or L3 and L5 (low UAC) to avoid the major aortic branches vessels (see Fig. 18.1 ). Complications of UACs include aortic thrombus, embolic events, and renal artery thrombus, which can result in hypertension or renal infarction.
Chest
How to Approach Lung Opacities in the Preterm Infant?
In the immediate neonatal period, particularly on the initial chest radiograph, surfactant deficiency syndrome is the most common diagnosis. Other diagnoses and superimposed conditions, such as infection, pulmonary edema, masses, and congenital anomalies, may also be present in preterm infants (see discussion in Chapter 19 ).
Respiratory Distress Syndrome
Respiratory distress syndrome (RDS) is the leading cause of death in babies who are born prematurely. RDS develops in approximately 10% of premature babies in the United States each year. RDS, now also known as surfactant deficiency syndrome, is the clinical manifestation of surfactant deficiency. Surfactant, a lipoprotein, synthesized by type II alveolar cells, lowers alveolar surface tension, thereby preventing alveolar collapse. Differentiation of type II alveolar cells occurs during the canalicular phase of lung development (18–28 weeks gestational age), after which surfactant is produced. Surfactant production increases with increasing gestational age. Alveolar development begins at 32 weeks gestational age and continues postnatally to 18 months of life.
Lack of sufficient surfactant in preterm infants results in increased surface tension within alveoli, and alveolar and acinar collapse. This radiographically manifests as low lung volumes, with granular opacification, and air bronchograms. The granular opacification that is typical of surfactant deficiency is secondary to collapse of alveoli with persistent aeration of the bronchioles and interstitial fluid secondary to capillary leakage. Radiographic findings are present shortly after birth and may progress in the first 24 hours of life ( Fig. 18.5 ).

The treatment of RDS involves prenatal administration of corticosteroids, respiratory support (continuous positive airway pressure and high-frequency oscillatory ventilation), and exogenous surfactant administration. Exogenous surfactant administration can result in symmetrical or asymmetrical improvement in lung aeration, possibly secondary to heterogenous exogenous surfactant distribution. A known complication of exogenous surfactant administration is pulmonary hemorrhage, which clinically manifests as respiratory decompensation after a period of clinical improvement. Radiographs will demonstrate new dense airspace consolidation ( Fig. 18.6 ).

Air Leak Complications in Respiratory Distress Syndrome
Air leak phenomena may develop in patients with RDS secondary to barotrauma and airway overdistension. It may occur in ventilated and nonventilated neonates. Air leak phenomena include pulmonary interstitial emphysema (PIE), pneumothorax, pneumomediastinum, and, rarely, pneumopericardium. PIE is the result of rupture at the bronchoalveolar junction, with subsequent tracking of air into the peribronchial and perivascular spaces. It may be focal or diffuse, and bilateral or unilateral. Radiographically, it manifests as branching lucencies, often cystic, along the expected course of the bronchovascular bundles ( Fig. 18.7 ). PIE detection is important as a warning sign for other air leak complications. Pneumothoraces, pneumomediastinum, and pneumatoceles may also occur as a sequela of barotrauma in patients with RDS, with or without PIE ( Figs. 18.8–18.10 ).