Encountering a neonatal imaging study may be an unsettling experience to radiologists who do not interpret them routinely. Normal structures often appear different from those of the adult or older child. Moreover, many differential diagnoses and disease processes are unique to the term neonate. In reality, however, there are a limited number of likely diagnoses that need to be considered for each imaging finding compared with the typically broader differential diagnosis that is generated for imaging findings in the adult or even older child. The accompanying medical history is brief. Finally, many disease states that affect the term neonate are based on altered anatomy that are amenable to detection on prenatal imaging. Because prenatal sonography is now commonplace, many diagnoses are no longer first made by postpartum imaging.
The goal of this chapter is to outline an approach to imaging the term neonate. It is broadly divided into two sections: the thorax and the abdomen. Discussion of disease entities and processes is organized around common imaging appearances. There is an emphasis on problem solving, both technical and interpretive. For further details regarding the relevant embryology, pathophysiology, and clinical aspects of term neonatal conditions, the reader is referred to several excellent articles and texts found in the Bibliography. A working knowledge of common findings encountered on neonatal imaging studies is essential to: (1) differentiate normal from abnormal; (2) accurately detect abnormalities; and (3) offer a concise differential or, when possible, a specific diagnosis.
Thorax
How Do I Know If My Radiograph Is Technically Adequate for Diagnosis?
The answer to this question depends in large part on what the clinical question is. Although every attempt should be made to acquire technically optimal images, poor technique should not blindly be used as “cover” to avoid interpreting aspects of the film that do carry diagnostic information.
In assessing technical adequacy, several observations should be made; these are discussed in the following subsections.
Collimation
Overcollimation is readily evident. Pertinent anatomy is excluded from the image. Conversely, undercollimation exposes extraneous anatomy to unnecessary ionizing radiation. In addition, the wider field of view increases scatter, decreasing image sharpness. In film/screen radiography, as well as in fluoroscopy, inclusion of very dense or very lucent regions (such as empty space outside the patient) increases the dynamic range, which falsely alters exposure parameters.
Projection/Angulation
It is simplest to image the newborn while supine, in anteroposterior (AP) projection. Fortunately, due to the geometry and small size of the infant thorax, there is no significant degradation of image quality or altered appearance of the mediastinum compared with the posteroanterior (PA) projection. As such, the AP projection is acceptable and widely used in the newborn and infant.
More problematic, however is unintended relative cranial angulation of the radiograph tube in relation to the chest, resulting in a lordotic projection ( Fig. 19.1 ). Due to the conical shape of the chest, a lordotic view often results when the baby lies flat. The effect is exaggerated when the arms are held back in extension and the midtorso is arched. To remedy this, a foam pad with a 15-degree incline (higher end toward the head) may be placed under the thorax. Alternatively, the tube may be angled slightly caudally. The appearance of lordotic positioning can be further exacerbated by a low centered beam, such as when acquiring a chest and abdomen on one cassette (“babygram”). In this instance the chest portion of the image is exposed by a more divergent beam. Ideally, the radiograph beam for a chest radiograph should be centered at the nipple line. The consequences of lordotic technique are outlined in Table 19.1 .

| The cardiac apex appears “uplifted,” simulating the appearance of right ventricular hypertrophy. |
| The anterior aspect of the first two or three ribs is projected at or above the remainder of the rib. |
| The clavicle is projected high above the lung apices. |
| Accentuation of bronchovascular markings and illusory airspace disease is most notable at the lung bases. |
| There may be loss of the sharp diaphragm as the diaphragm is now imaged more en face . |
Positioning
A well-positioned patient is judged on a frontal radiograph by assessing the medial heads of the clavicle. The sternoclavicular junction should be an equal distance between left and right. In addition, the rib lengths should be symmetrical. When these findings are not met, the patient is rotated about the vertical axis. Spurious relative hyperlucency of one hemithorax is most commonly a result of simple patient rotation. Rotation-related changes in projection of mediastinal structures are particularly pronounced in children, largely because of the thymus. As a relatively bulky anterior chest structure, a small degree of patient rotation results in substantial differences in its projection ( Fig. 19.2 ). Indeed, this phenomenon can lead to a spurious impression of cardiomegaly or even upper lobe consolidation. Rotation uncovers the hilus of the side opposite the direction of rotation, resulting in accentuation of these structures (most typically on the left). With rotation, individual sternal ossification segments can appear as lung nodules or even as rib calluses ( Fig. 19.3 ).


Respiration
In contrast with adults or older children, neonates and young children are imaged while freely breathing. Thus exposure time must be kept to a minimum to avoid motion blur. Just as important, though, is the phase of respiration that the image is acquired in. This is, of course, most challenging in a neonate because the normal neonatal respiratory rate is 20 to 60 breaths/min. Ideally, the image is acquired near peak inspiration. Attempted imaging during peak inspiration requires carefully observing the rise and fall of the chest and abdomen to synchronize the exposure at or close to peak inspiration. As a rule of thumb, on the frontal projection, six anterior ribs should be seen above the diaphragm ( Fig. 19.4 ). In infants, counting anterior ribs is more reliable due to the more anterior dome of the diagram. Better yet is an overall assessment, including the degree of flattening of the diaphragm because other variables such as parallax as a result of positioning, will vary where the rib crosses the diaphragm.

Spurious findings commonly present because of inadvertent exposure during expiration are outlined in Table 19.2 . When carefully examined, the findings could be suggested to be due to technique, although differentiation from true pathology may be tricky. For example, the hazy increased density seen on an expiratory film may not seem to be made up from acinar infiltrates, nor does it appear as a result of reticular densities. Rather, there is a generalized haze and a crowded appearance to the hilar vessels. It may be erroneous, however, to confidently ascribe such findings to technique. This is even more challenging in neonates where there is much overlap already between different patterns of disease on the chest radiograph. Most importantly, true findings may be missed entirely on an expiratory film. For example, focal lung disease may be “hidden” by a high diaphragm or may be rendered inconspicuous by the already generally increased lung density that decreases contrast in the image.
| Cardiomegaly |
| Pulmonary edema |
| Overcirculation |
| Multifocal pneumonia |
| Illusory posterior mediastinal soft tissue |
Exposure
Arguably, in the digital era, exposure factors have become less crucial to set with the same degree of precision as in the past. This is due to the much wider latitude digital radiography affords because it can record a far wider dynamic range than can film/screen. Still, setting proper exposure parameters is important for several reasons. First, the dynamic range is impressive but not infinite. At the extremes of density (such as dense, overlapping osseous structures and aerated lung), there still can be loss of detail that is not recoverable by adjusting the window and level settings on the display monitor ( Fig. 19.5 ). In addition, regardless of overall exposure, the inherent contrast is still affected by exposure parameters such as kilovoltage peak (kVp). Importantly, an underexposed film can usually be brought back to the “correct” contrast on the display panel; however, the resulting increased noise in the film from quantum mottle renders a suboptimal image. This underexposure then imparts a “grainy” or hazy appearance to the image. When interpreting such a film, examining the soft tissues for these effects will help the reader differentiate this phenomenon from true lung pathology.

Within a large range of exposure values, overexposure in digital radiography may not compromise overall image quality. Because it is not visually readily evident, this actually creates a problem as there is no longer a feedback to the technologist to keep the dose in check. Whenever there is a quality issue, the dose can easily be raised to compensate for another deficiency. It is said that the exposure and final image product have been “decoupled.” This results in ever-increasing radiation doses, known as “dose creep,” and is something that both the technologist and radiologist should be aware of to adhere to the as low as reasonably achievable (ALARA) principle.
In addition to underexposure, the final image is affected by postprocessing techniques, such as use of an unsharp mask filter, or edge enhancement or changes in the gradient shift (see Fig. 19.2C ). This can adjust the conspicuity of fine linear structures, including catheters, fissures, and the vascular markings. A detailed discussion of this, as well as other postprocessing techniques, is beyond the scope of this book.
Visualization of the lower vertebral endplates and pedicles through the mediastinal structures on a frontal radiograph suggests proper exposure. In addition, vascular markings should be easily seen in the central two-thirds of the lungs.
Is That Normal?
For the thoracic cage, the cliché “children are not just small adults” is as true in neonatal chest radiology as it is in much of pediatric medicine. To those unaccustomed to interpreting pediatric chest images, the appearance of the pediatric chest can be strange. Moreover, as discussed earlier, its appearance can change from one study to the next because small differences in patient and radiograph tube positioning can have a dramatic effect on the radiograph appearance. The normal full-term newborn thorax has a “lamp-shade” appearance on the frontal view. The ribs are relatively horizontal in configuration and develop the downsloping appearance later on in infancy. The bones can appear relatively dense (neonatal sclerosis). Exaggeration of the horizontal configuration and upsloping of the first two or three anterior ribs may indicate inadvertent lordotic positioning.
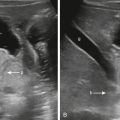
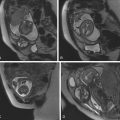
Congenital masses of the chest wall are rare. Mesenchymal hamartoma of the chest wall is a benign rib mass with an ominous appearance on chest radiograph that shows relatively characteristic mass effect, rib erosion, calcification, and soft tissue ( Fig. 19.6A ). Magnetic resonance imaging (MRI) shows heterogenous signal because the histology consists of prominent cartilaginous tissue with hemorrhagic cysts (see Fig. 19.6C ). Either partial or complete resection is recommended because there may be respiratory compromise and because there are case reports of malignant congenital tumors of the chest wall.

Humeral Ossification Centers
Presence of the proximal humeral ossification centers strongly suggests that the newborn is greater than 38 weeks of gestation ( Fig. 19.7 ). The converse, however, is not true. That is, the humeral ossification centers are not always present yet in the full-term infant (see Fig. 19.4 ). Moreover, the appearance of the proximal humeral ossification centers, as well as the coracoid ossification center, is variably delayed in different populations.

Broken Clavicle
At delivery, occasionally a clavicle fracture may occur. Radiographs are often obtained to document and confirm the fracture ( Fig. 19.8A ). Not all broken-appearing clavicles are fractured. When the ends appear rounded and corticated, the possibility of a congenital pseudoarthrosis of the clavicle should be raised (see Fig. 19.8B ). Similar to clavicular birth fractures, these may present with soft tissue swelling and, occasionally, reduced movement of the arm. It is most often seen on the right side.

The appearance of pseudoarthrosis is distinct from the appearance of the clavicle in patients with cleidocranial dysostosis. Cleidocranial dysostosis is a disorder that affects intramembranous ossification and, to a lesser extent, endochondral ossification. As such, patients with cleidocranial dysostosis will present with hypoplastic or aplastic clavicles (see Fig. 19.8C ). Common coexisting radiographic abnormalities include wormian bones and absence of ossification of the symphysis pubis (see Fig. 19.8D ).
Tracheal Buckling
Not only is lateral and anterior tracheal buckling normal in the neonate, its presence actually assists in determining the side of the aortic arch. The typical convex rightward buckle of the trachea is consistent with a normal left-sided aortic arch. A leftward buckle is expected with a right-sided aortic arch. The buckling is more pronounced during expiration or with the head turned. It can give the false impression of a mass on the frontal view or prevertebral soft tissue thickening on the lateral view. If there is any uncertainty, repeating the radiograph with attention to head positioning and respiratory phase may confirm the buckling to have been only transient.
Troubleshooting a Narrowed-Appearing Airway
The newborn trachea often has a narrowed appearance. It is said that there can be up to 50% narrowing of the trachea between inspiration and expiration in adults and children. In newborns, however, the airway narrowing in asymptomatic patients during the expiratory phase can be even more profound. Nonetheless, an AP and lateral study of the airway during fluoroscopy may be of benefit in selected patients who have clinical evidence of stridor. Unfortunately, the fluoroscopic findings of the normal overlap with those of tracheomalacia. However, the following generalizations may be helpful: (1) a focal expiratory collapse of the trachea is not normal and can suggest a vascular ring, (2) collapse of the trachea in the context of stridor suggests tracheomalacia, (3) a normal study does not exclude bronchomalacia, and (4) laryngomalacia is actually the most common cause of infantile stridor and will not be evident on routine radiological airway studies.
Multidetector computed tomography (CT) is more reliable than fluoroscopy. On CT, the caliber of the subglottic trachea may be used as an internal reference because it does not contain tracheal cartilage. Three-dimensional reconstructions of the airway are invaluable. More recently, some centers have been using real-time multidetector CT evaluation of the airway played back as cine clips, which is advantageous when the finding is transient. Most infants will instead undergo direct visualization under bronchoscopy for definitive diagnosis.
Tracheomalacia is divided into primary and secondary types. Primary tracheomalacia can occur in premature infants or in several systemic cartilaginous disorders, such as relapsing polychondritis and Larsen syndrome. Secondary tracheomalacia occurs in patients with tracheoesophageal fistula (TEF) (commonly), extrinsic vascular compression, and mediastinal soft tissue masses.
Tracheal stenosis is distinct from tracheomalacia and is differentiated by tracheal narrowing throughout the respiratory cycle and biphasic stridor. Long-segment tracheal stenosis is nearly synonymous with complete tracheal rings, an anatomic anomaly where the rings are less pliable because they lack the normal posterior membranous portion. A low position of the carina with horizontally positioned mainstem bronchus strongly suggests the condition ( Fig. 19.9A ). The trachea may appear consistently narrow on each chest radiograph. Although direct bronchoscopic visualization is diagnostic, a profoundly narrowed lumen may not allow passage of the bronchoscope to the most distal narrowed segment, which may include the bronchi. In such case, CT scan may be obtained to detect bronchial involvement, which can establish surgical correctability. Approximately one-third of patients with tracheal stenosis/complete tracheal rings also have an aberrant left pulmonary artery arising from the right main pulmonary artery (type 2 pulmonary sling) (see Fig. 19.9B ).

Short-segment stenosis (fewer than five rings involved) may be surgically corrected with resection and primary end-to-end anastomosis. Increasingly used techniques for the longer stenotic segments include the “slide tracheoplasty” and the use of tracheal stents.
Heart and Mediastinum: Is It Enlarged? Or Is It All Thymus?
The variability in the size and orientation of the thymus from one patient to the next and even from one radiograph to the next in the same patient renders cardiothoracic ratios limited in the full-term newborn, particularly when the image is acquired in expiration. A rule of thumb is that the transverse cardiothoracic ratio should be less than 60%. An attempt should still be made to assess overall heart size and shape on the frontal radiograph, but the practice is a subjective one. The lateral view allows an unobstructed view of the posterior border, which should not extend to the spine.
There are several additional structures that may be normally seen on a neonatal chest radiograph that would be considered abnormal in an older child or adult. This includes occasional visualization of the right edge of the left atrium on the AP view, which projects to the right of the spine, near the upper heart border. Another finding considered normal is the “ductus bump.” This typically represents the partially patent or closing ductus as the pulmonary pressures fall below left heart pressure.
One should attempt to assess the inferior margin of the thymus, particularly on the left. The extent of the cardiomediastinal silhouette that extends below this point provides an unobstructed view of a segment of heart border. This margin is often demarcated by a focal “notch” (see Fig. 19.2D ). Cardiac border can often be differentiated from thymus by the presence of the so-called “thymic wave”. This describes subtle undulation of the soft tissue border caused by the impression from the anterior ribs on the soft thymic gland. When the thymic gland is small, such as in the stressed state of an ill infant, or entirely absent, such as in DiGeorge syndrome, the cardiac contours and central vessels are better visualized. Further details regarding findings in congenital heart disease are covered in Chapter 3 .
On a lateral view, obscuration of the retrosternal clear space in an infant is a normal finding because of the anterior mediastinal position of the thymus.
At times, the thymus can appear so large and irregular as to suggest the presence of a mass or vascular anomaly of the great vessels ( Fig. 19.10 ). For example, a bulky thymic shadow can mimic the superior mediastinal fullness seen in supracardiac anomalous pulmonary venous return or mimic the appearance of a soft tissue mass. If there are any red flags, such as mass effect on an adjacent structure (such as the airway), bone changes, or calcification, further investigation by cross-sectional imaging is mandatory. In the absence of such findings but still with a concern for a mediastinal abnormality, there are several approaches for further workup. First, review of any available prenatal imaging may prove helpful. Second, if it is believed that the image in question was captured in expiration, simply repeating the radiograph may be all that is required.

If uncertainty persists, fluoroscopy of the chest can show the expected change of size and shape of a normal thymus with respiration. Ultrasound is a more direct method for visualizing the thymus. This thymus appears as hypoechoic soft tissue with numerous foci of hyperechoic reflections creating a speckled or “starry sky” pattern (see Fig. 19.10D ). Its margins will be seen to alter with respiration. The posterior extent of the thymus should be imaged to where it interfaces with lung. On a neonate, this is a relatively short distance. In addition, the sternum offers a better acoustic window in the neonate because of nonossified cartilage. As a result, even the posterior extent of the gland is readily visualized by ultrasound. Common variants include thymic extension behind the superior vena cava or superior extension into the neck, and ectopic locations in the anterior mediastinum and neck—all readily identifiable by ultrasound (see Fig. 19.10 ).
MRI, because of its exquisite soft tissue contrast, can confidently confirm the presence of a uniform thymus and exclude a pathological mass. However, because of its higher cost, less availability, risk for sedation (when needed), and potential artifacts, it can be reserved for cases that are unclear by ultrasound alone. For further discussion and additional images of the thymus, please see Chapter 1, Chapter 2 .
Pitfalls in Imaging Lungs: Normal Findings
Several accessory fissures may be visible on a chest radiograph. A fissure is a visceral pleural-lined cleft extending into the lung to varying depths and is often incomplete. The azygos fissure and accessory inferior pulmonary fissure are common examples. The latter is typically seen vertically at the right base, separating the medial basal segment from the remaining right lower lobe basilar segments. The major fissure is typically not apparent on a frontal chest radiograph; however, with slight rotation, it may become visible ( Fig. 19.11 ). Its appearance is most often seen in infants with cardiomegaly, probably because of resulting slight rotation of the right lower lobe. These fissures may be confused with atelectasis, scarring, a skin fold, or the pleural line of a pneumothorax.

A retrosternal triangular density seen inferiorly on some lateral chest radiographs is thought to reflect a normal mediastinal soft tissue interface that extends more anteriorly than the left lung ( Fig. 19.12 ).

The appearance of an ovoid vertically oriented lucency in newborns, mimicking the dilated esophagus of esophageal atresia (EA), is not uncommon ( Fig. 19.13 ). This lucency is caused by the suprasternal fossa, a depression on the skin between the sternal heads of the sternocleidomastoid muscle and should not be confused with a dilated esophagus or trachea. It is more common in the setting of respiratory distress due to recruitment of these accessory muscles of respiration.

Approach to Pulmonary Vascularity
The newborn lungs often appear slightly more hyperlucent on radiograph than that of the older child. This is both due to the smaller-caliber vessels and the geometry of the newborn chest. Indeed, one of the most difficult aspects of pediatric chest radiograph interpretation is evaluation of the pulmonary vasculature. Simply stated, this is an assessment of vascular “plethora” versus decreased pulmonary vasculature. In practice, the findings are often subjective and may be influenced by variations in radiographic technique. A study by Tumkosit et al. (2012), using cardiac catheterization as the gold standard, did show reasonably good accuracy when the reader interprets shunt vascularity on frontal chest radiographs in children. The same study, however, showed poorer sensitivity in detecting mildly decreased pulmonary vascularity. The term “plethoric” vasculature reflects both the increased width of the vessel and the impression of “too many vessels.” For the size of the vessel, it is useful to compare a vessel on end with an adjacent bronchiole, if seen. Another technique is to compare the diameter of the trachea to the right descending pulmonary artery, which should nearly equal in caliber.
This increased vascularity should not be confused with the physiology of venous hypertension, as seen with left-sided obstructive cardiac lesions or obstructed pulmonary venous return. Such findings appear more typical of interstitial edema, such as “fuzzy” margins of vessels and central hazy densities. In practice, this pattern may be identical to that of retained fetal fluid, and there is an overlap in appearance with other entities, including neonatal pneumonia. Even differentiating edema from shunt vascularity on many chest films can be challenging and, sometimes, an impossible task. In fact, with enough overcirculation, edema will result.
For the first several hours to days of life, pulmonary vascular resistance is still elevated. As such, most intracardiac shunt lesions will not be apparent on the neonatal chest radiograph. To make matters more complex, persistent fetal hypertension (also known as persistent pulmonary hypertension of the newborn) can persist beyond the newborn period, either because of lung hypoxia, acidemia, structural heart disease, or as an idiopathic condition. Its physiological hallmark is persistence of the fetal right-to-left shunting, which typically renders the vascular pattern on the chest radiograph either normal or decreased. The degree of hypoxemia in the newborn with this condition may be profound, but the lungs appear clear. When underlying structural heart disease needs to be excluded as a cause, it is usually accomplished with cardiac echo and is at this point beyond the capability of the chest radiograph. A more detailed discussion of the differential diagnosis of congenital heart disease can be found in Chapter 3 .
General appearances and patterns of the abnormal lung parenchyma are described in the following section.
Diffuse Granular Hazy Appearance: What Should I Be Thinking Of?
A newborn lung with a ground-glass, granular, hazy pattern, with or without air bronchograms, typically evokes the diagnosis of respiratory distress syndrome of the premature infant due to surfactant deficiency, which is covered in detail in Chapter 18 . Surfactant deficiency in the full-term or near-full-term infant is uncommon but can occur in the setting of gestational diabetes. Less common still are genetic disorders of surfactant causing congenital deficiency irrespective of the gestational age ( Fig. 19.14 ).