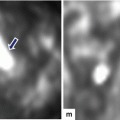
Fig. 5.1
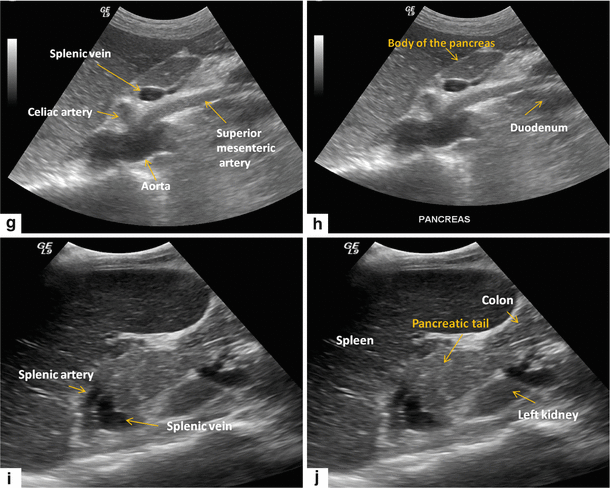
Normal anatomy of the pancreas on transabdominal ultrasound. Transverse (a, b) and sagittal sonograms obtained progressively from paramedian region toward the splenic hilum (c–j) show the vascular landmarks (a, c, e, g, i), and the anatomic divisions of the pancreas and adjacent organs (b, d, f, h, j)
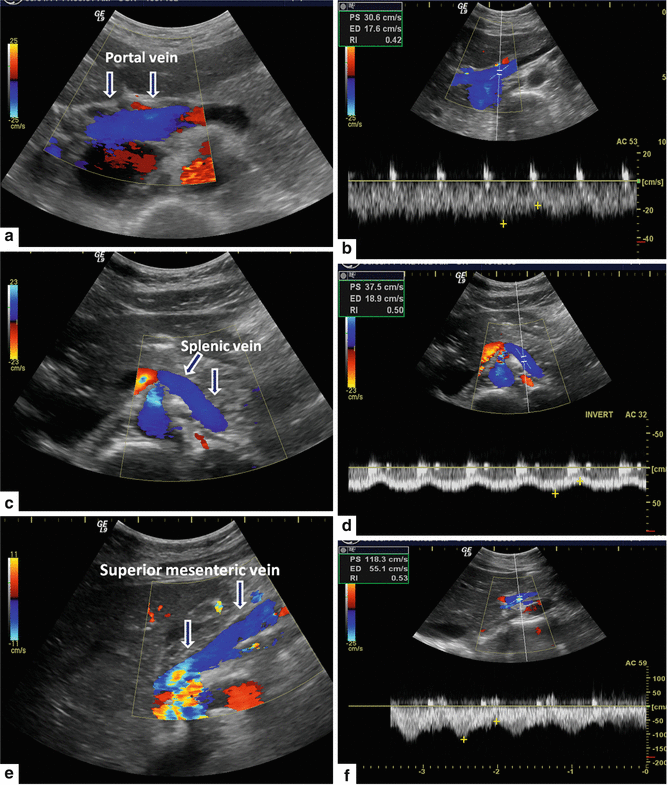
Fig. 5.2
Color Doppler of the portal vein system. (a) Portal vein, transverse scan. This vessel arises from the junction of the splenic and superior mesenteric vein posterior to the pancreatic neck and courses obliquely to the right to terminate in the porta hepatis (arrows). (b) By Doppler, this vein demonstrates hepatopetal flow with monophasic, low velocity waveform which undulates slightly with respiration. (c) Splenic vein, transverse scan. This vein runs posterior to the body and tail of the pancreas from the splenic hilum to the neck of the pancreas (arrow). (d) By Doppler, this vein demonstrates hepatopetal flow with monophasic, low velocity waveform which undulates slightly with respiration. (e) Superior mesenteric vein, sagittal scan. This vein runs parallel and to the right of the superior mesenteric artery (arrows). It joins to the splenic vein posterior to the neck of the pancreas. (f) By Doppler, this vein demonstrates hepatopetal flow with monophasic, low velocity waveform which undulates slightly with respiration
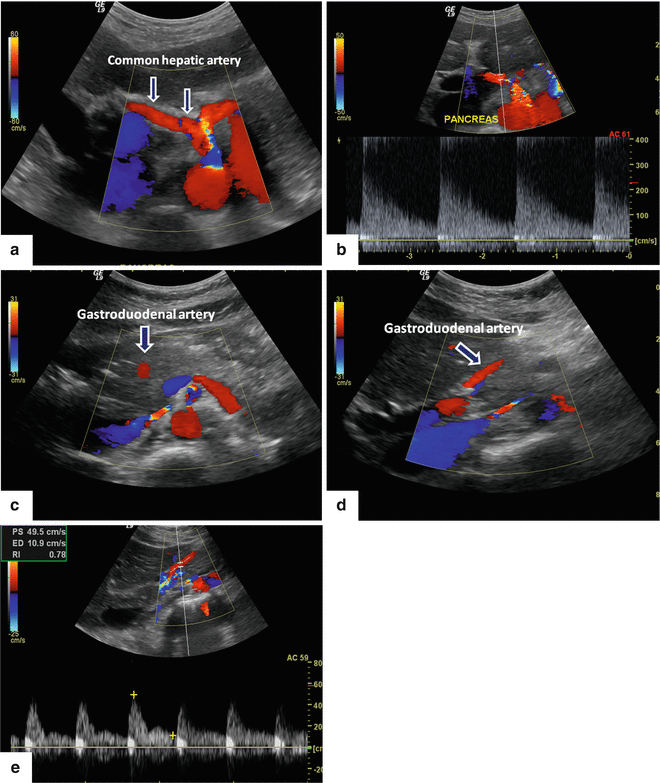
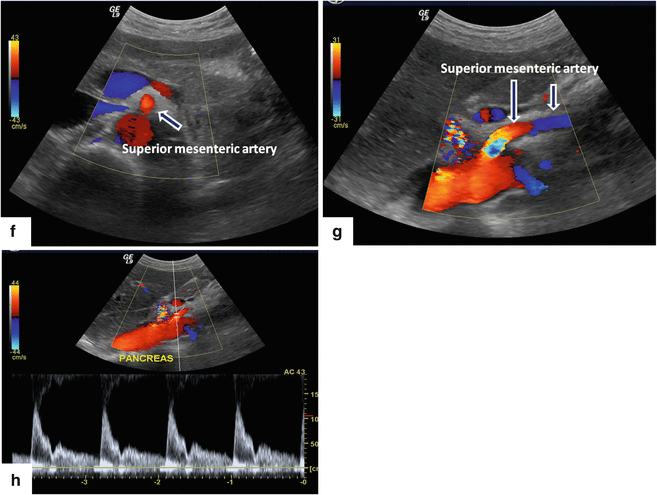
Fig. 5.3
Color Doppler of the main arteries of the pancreas. The hepatic artery on color Doppler, transverse scan (a). The common hepatic artery (arrows) after arising from the celiac artery advances a short distance along the superior border of the pancreatic head (arrows) and then divides into the hepatic proper and the gastroduodenal artery. By Doppler ultrasound, this vessel shows a low-resistance arterial signal (b). Gastroduodenal artery on color Doppler transverse and sagittal scans (c, d). This artery after arising from the common hepatic artery can be identified in the anterosuperior border of the pancreatic head (arrow). By Doppler ultrasound, this artery shows the same low pulsatility signal as the hepatic artery (e).
Superior mesenteric artery on color Doppler transverse and sagittal scan (f, g) (arrows). This vessel arises from the aorta immediately below the origin of the celiac artery. It can be identified between the aorta and the splenic vein. On Doppler ultrasound, this artery shows a low-resistance diastolic flow (h). Note: 30–90 min after eating, the flow in this artery changes to a high resistance waveform
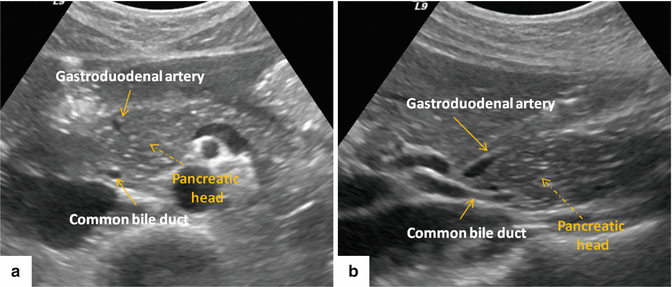
Fig. 5.4
Transverse (a) and sagittal (b) views of the head of the pancreas show the anatomic location of the gastroduodenal artery and common bile duct
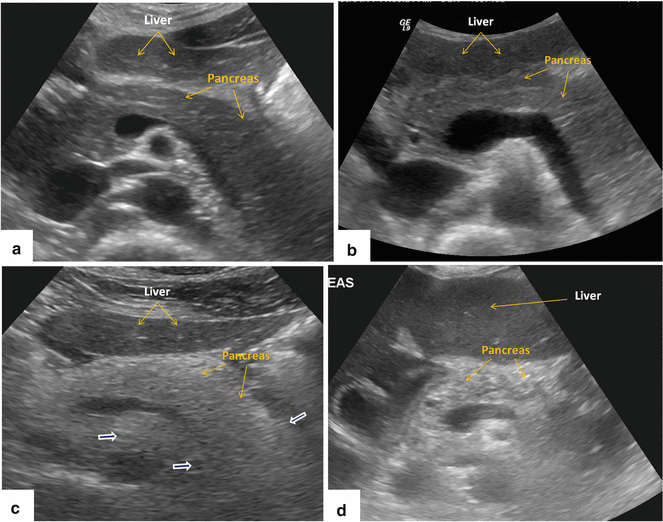
Fig. 5.5
Variants of normal pancreatic echogenicity. (a) Transverse scan of the pancreas of a 35-year-old male patient shows that the pancreatic parenchyma (arrows) has similar echogenicity to the liver parenchyma (arrows). (b) Transverse scan of the pancreas performed on a 22-year-old female patient shows that the pancreatic parenchyma (arrows) is more echogenic than the liver parenchyma (arrows). (c) Transverse image of the pancreas of a 73-year-old female shows a homogeneous increase of echogenicity of the pancreas (arrows) similar to the retroperitoneal fat (white arrows). (d) Transverse image of the pancreas of a 68-year-old male shows a heterogeneous increased echogenicity of the pancreas (arrows)
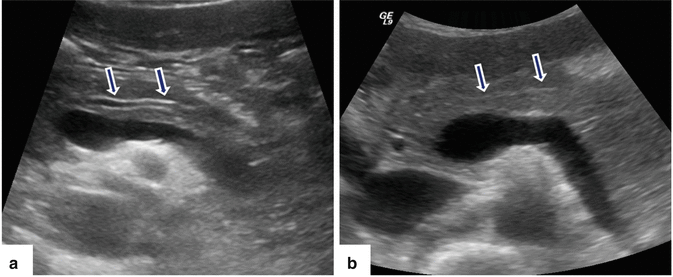
Fig. 5.6
Normal appearance of the main pancreatic duct in two different patients on conventional ultrasound. (a) Transverse image of the pancreas shows the main pancreatic duct appearing as two parallel echogenic lines (arrows). (b) Transverse image of the pancreas shows the main pancreatic duct appearing as a single echogenic line (arrows)
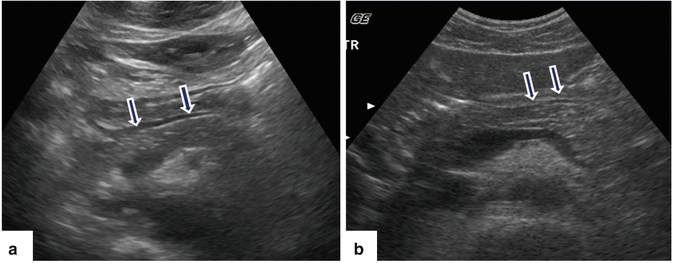
Fig. 5.7
Pseudopancreatic duct on conventional ultrasound in two different patients. Transverse views (a, b) of the body of the pancreas show a tubular structure with parallel echogenic lines (posterior wall of the stomach) mimicking a pancreatic duct (arrows)
The pancreas can be localized by ultrasound by identifying its parenchymal architecture and the adjacent anatomic landmarks.
The most useful landmark to identify the pancreas is the splenic vein (SV).
On transverse scan, the splenic vein appears as a tubular anechoic structure located directly posterior to the body and tail of the gland.
The confluence of the splenic and superior mesenteric vein (SMV) occurs just posterior to the pancreatic neck.
The superior mesenteric vein courses between the pancreatic neck and uncinate process.
The portal vein (PV) courses cephalad to the hepatic hilum.
The splenic artery (SA) runs in the superior border of the body and tail of the pancreas. This artery is often markedly tortuous.
The superior mesenteric artery runs posterior to the superior mesenteric vein.
The gastroduodenal artery (GDA) courses along the right anterolateral aspect of the pancreatic head. This structure appears as a small anechoic dot on a transverse view.
The normal pancreatic parenchyma usually has a homogeneous texture and is isoechoic or hyperechoic to the normal liver parenchyma.
With age, fatty replacement of the pancreas is common, and the parenchyma may appear as echogenic as the adjacent retroperitoneal fat or may adopt a heterogeneous appearance.
The pancreatic duct may be seen as a double parallel echogenic line or as a single linear echogenic structure.
The main pancreatic duct can be confused with the posterior wall of the stomach (pseudopancreatic duct).
The common bile duct lies in the posterior aspect of the head of the pancreas.
It appears as a small anechoic dot in the inferior lateral aspect of the head of the pancreas in the transverse plane and as a tubular structure in the posterior aspect of the head of the pancreas on the sagittal plane.
The average diameter of the common bile duct on ultrasound is 7 mm and no larger than 10 mm after cholecystectomy.
Practical Pearls
Ultrasound is an extremely useful screening method for evaluating patients with jaundice to determine the presence of biliary obstruction. However, in order to accurately identify the site and the etiology of the biliary obstruction, computed tomography or magnetic resonance is required.
In patients with acute pancreatitis, ultrasound is commonly utilized to look for biliary stones, fluid collections, or vascular complications.
5.2.1.2 Endoscopic Ultrasound (EUS) (Figs. 5.8 and 5.9)
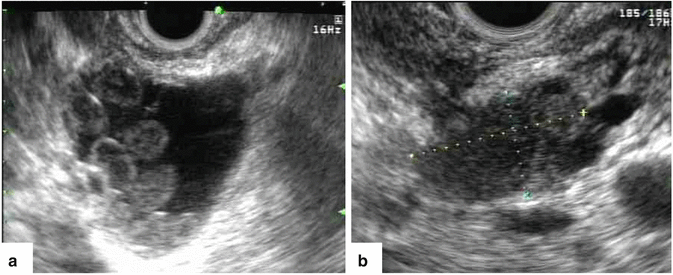
Fig. 5.8
Endoscopic ultrasound (EUS) in two different patients with indeterminate pancreatic lesions by computed tomography. (a) EUS transverse view demonstrates a complex mass with cystic and solid elements (FNA: borderline IPMN). (b) EUS transverse view reveals a solid mass in the head of the pancreas (FNA: malignant IPMN)
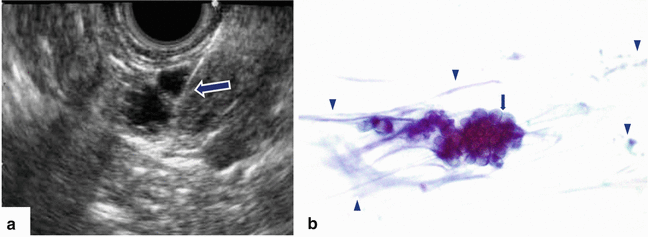
Fig. 5.9
Guided fine-needle aspiration using endoscopic ultrasound (EUS). (a) Transverse view shows a needle (arrow) within a complex cystic mass. Cytology specimen (b) shows a cluster of cohesive cells with well-defined borders and increased mucin-producing cytoplasm (arrows) floating in a background of mucin (arrowheads). (Papanicolau, 60×)
During EUS, the entire pancreas can be examined in almost all patients, as gas and fat interferences are eliminated.
The great advantage of this technique is that in addition to the images, tissue samples can be obtained in real time using fine-needle aspiration techniques (EUS-FNA).
Indications
Early detection of small pancreatic carcinoma
Staging pancreatic malignancy
Detection of neuroendocrine tumors
Characterization of cystic pancreatic masses
Limitations
Availability, a small field of view, requires patient sedation.
EUS–FNA complications
Acute pancreatitis, hemorrhage, and infection
Practical Pearl
This imaging technique is especially useful for detecting and characterizing small pancreatic masses; however, it requires an expert endoscopist, and therefore availability of this technique is limited in many medical centers.
5.2.1.3 Intraoperative Ultrasound (IOUS) (Figs. 5.10–5.13)
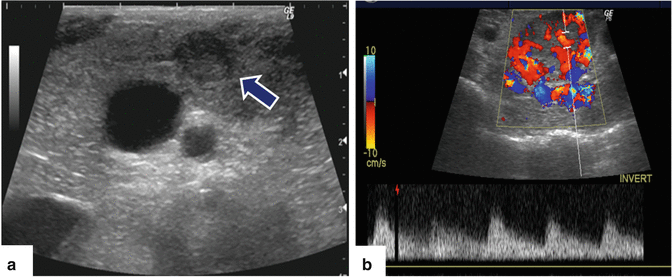
Fig. 5.10
Intraoperative ultrasound of the pancreas, performed for localizing an undetectable insulinoma by computed tomography and magnetic resonance. (a, b) IOUS transverse image show a small (a) (arrow) hypoechoic, hypervascular mass in the body of the pancreas. Pathology: insulinoma (6 mm in diameter)
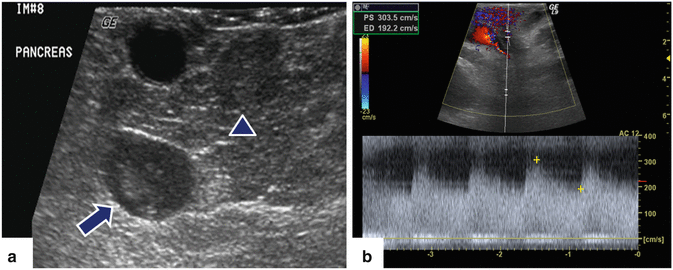
Fig. 5.11
Intraoperative ultrasound in a patient with MEN-1 syndrome with a small neuroendocrine tumor in the head of the pancreas detected by computed tomography. (a, b) IOUS demonstrates a small hypoechoic, hypervascular mass in the pancreatic head (arrow) and an additional second lesion in the pancreatic neck (arrowhead)
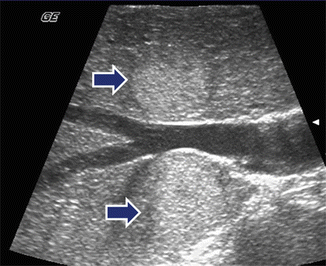
Fig. 5.12
Intraoperative ultrasound (IOUS) of the liver performed for detection of hepatic metastases in a patient with known pancreatic neuroendocrine tumors. Transverse scan of the liver demonstrates two hyperechoic solid lesions in segments 7 and 8 of the liver (arrows)
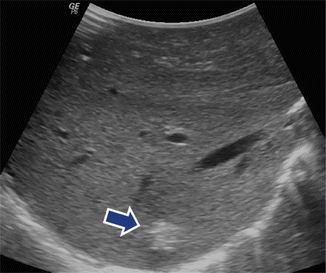
Fig. 5.13
Patient with a malignant neuroendrocrine tumor of the pancreas who underwent an IOUS of the liver to rule out metastases. Transverse scan of the liver displays a single lesion in segment seven of the liver (arrow). This lesion was not detected by MDCT. A segmentectomy was performed. Final pathology revealed a single metastatic deposit
Intraoperative ultrasound (IOUS) is a dynamic imaging modality that provides interactive and timely information during surgical procedures.
Because the tranducer is in direct contact with the organ, high resolution images can be obtained that are not degraded by air or overlying soft tissues.
It can accurately localize pancreatic pathology, hepatic metastases, limit the extent of surgical resection, improve surgical staging, guide surface incisions for deep lesion resection, and may help the surgeon to select the optimal surgical procedure.
Indications
Staging and localizing pancreatic tumors
Performing regional metastatic surveys
Documenting arterial and venous patency
Identifying endocrine tumors
Distinguishing pancreatitis from a neoplasm
Guiding biopsy, duct cannulation, and to drain abscesses
Limitations
Availability, time consuming, and operative dependent
Practical Pearl
Intraoperative ultrasound has proven to be extremely useful in identifying small pancreatic neuroendocrine tumors not seen by the other imaging modalities or palpable by the surgeon. It can detect small metastatic lesions deep within the hepatic parenchyma.
5.2.2 Multidetector Computed Tomography
Multidetector computed tomography (MDCT) is the most commonly used imaging modality for the evaluation of pancreatic pathology.
MDCT has improved volume coverage, speed, and spatial resolution along the z-axis
It allows quick multiplanar reconstructions (MPR) or three-dimensional reformatting of the pancreas with the use of isotropic voxels: shaded surface display (SSD), maximum intensity projection (MIP), and three-dimensional volume rendering (3D VR).
MDCT is the modality of choice for the initial diagnosis and staging of pancreatic malignancies.
MDCT permits the acquisition of images in the arterial, pancreatic, and portal venous phases with a delay of 20, 40, and 70 seconds, respectively.
Most pancreatic neuroendocrine tumors and their metastases are small and very vascular and would be best seen in arterial phase images (20 s).
Maximum enhancement of the pancreas and the maximum tumor-to-parenchymal attenuation is achieved during the pancreatic phase (40 s).
The sensitivity improves if images are obtained at 70s in the portal venous phase.
Indications
Initial diagnosis and staging of pancreatic masses.
Diagnosis or confirmation of acute/chronic pancreatitis and its complications.
Determine the extension and location of peripancreatic fluid collections and vascular complications.
This imaging modality is as useful as with magnetic resonance for characterizing pancreatic cystic lesions.
Dynamic contrast-enhanced CT is the mainstay of imaging in suspected traumatic pancreatic injury.
This modality is the preferred guidance method for percutaneous needle biopsy of pancreatic masses and for aspiration or percutaneous drainage of pancreatic collections.
Disadvantages of CT
Ionizing radiation
Use of intravenous contrast
Normal pancreas on MDCT
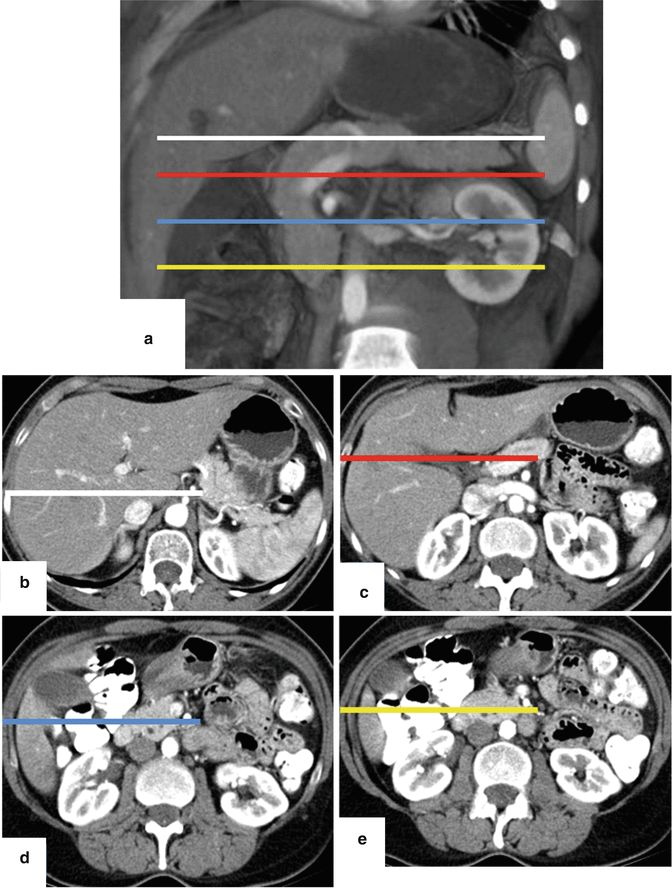
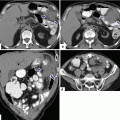
Fig. 5.14
Evaluation of the pancreas by MDCT. Volume rendered coronal image of the pancreas (a) shows the oblique orientation of this organ in the retroperitoneum (colored lines). Conventional axial images show that the pancretic tail (b) is located higher at level of T2 (white line), than the pancreatic body (c) at the level of L4 (red line), than the head (d) at the level of C1-L2 (blue line) and than the uncinate process (e) at the level of L2 (yellow line)
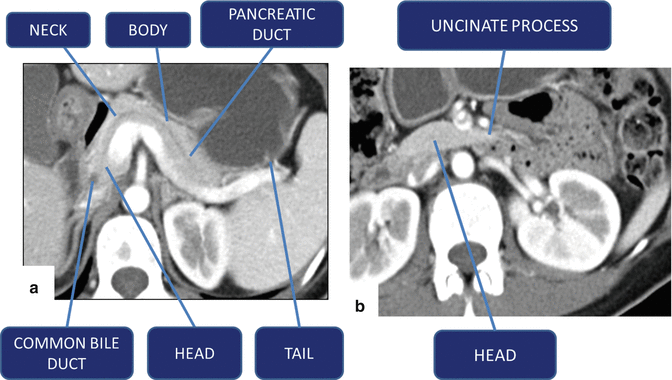
Fig. 5.15
Pancreatic divisions on contrast-enhanced CT (a, b)
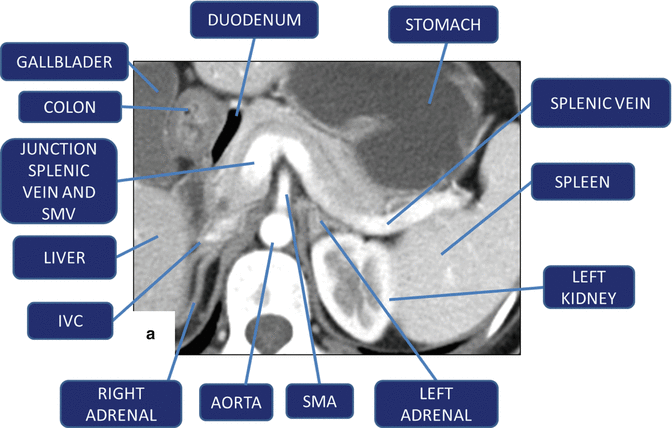
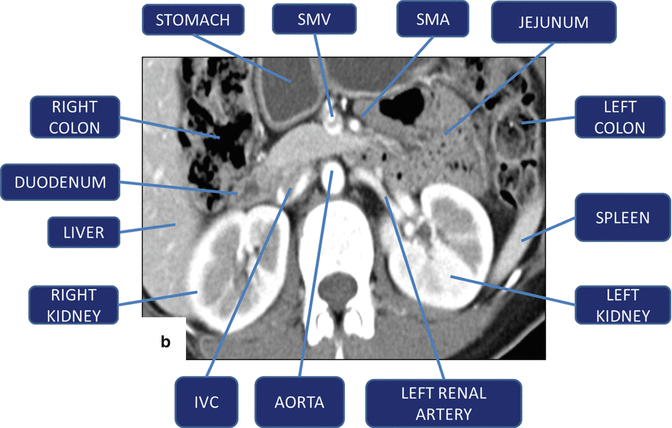
Fig. 5.16
(a, b) Vascular landmarks of the pancreas and neighboring structures on contrast-enhanced CT axial images
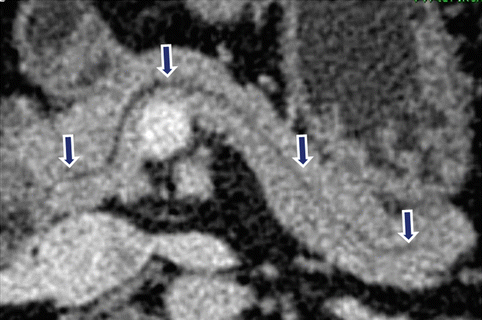
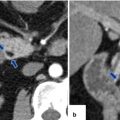
Fig. 5.17
Normal pancreatic duct on CT. Axial contrast-enhanced CT with curved planar reformat shows the entire length of the pancreatic duct (arrows)
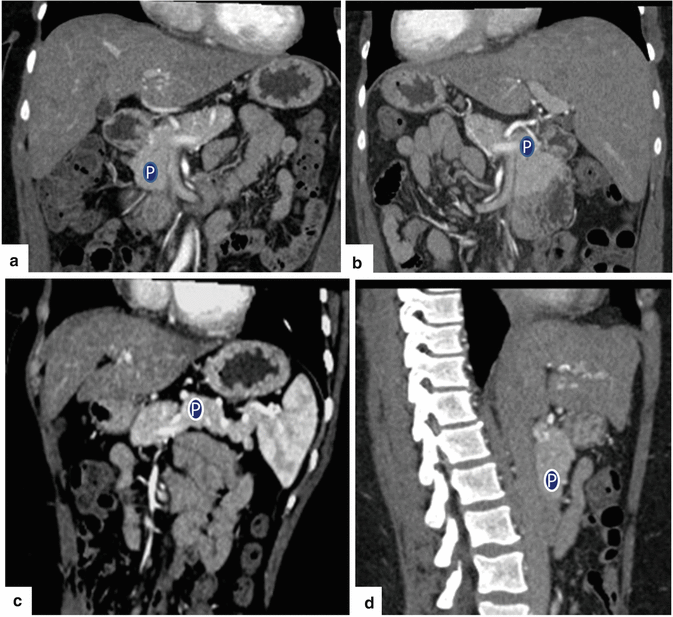
Fig. 5.18
Multiple planar reformats of the pancreas (P) on contrast-enhanced CT. (a) Anterior coronal plane. (b) Posterior coronal plane. (c) Oblique plane. (d) Sagittal plane
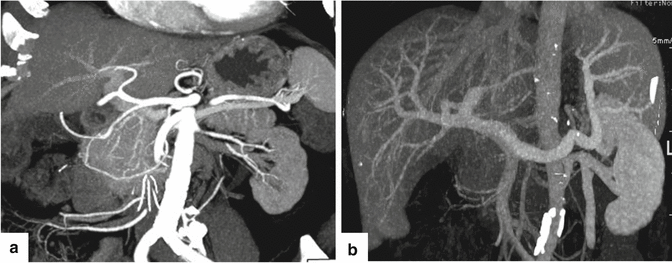
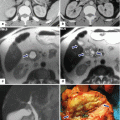
Fig. 5.19
CECT maximum intensity projection (MIP) reformation. Coronal images demonstrate the main arteries that supply the pancreas (a) and the main veins that drain the pancreas (b)
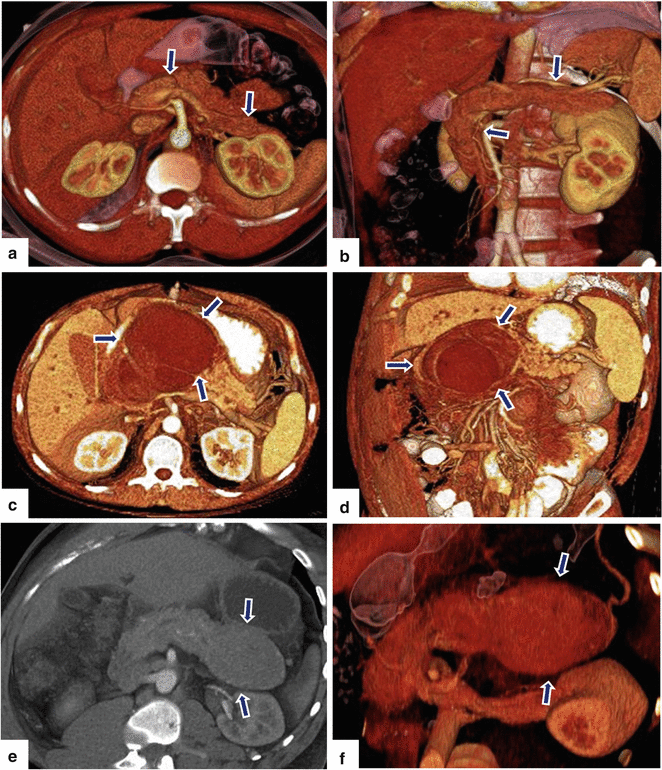
Fig. 5.20
3D volume-rendered reformats of the pancreas on contrast-enhanced CT. (a, b) Axial and coronal images show a normal pancreas (arrows). (c, d) Axial and coronal images show a pancreatic pseudocyst (arrows). (e, f) Axial and coronal images show a neuroendocrine tumor on the tail of the pancreas (arrows)
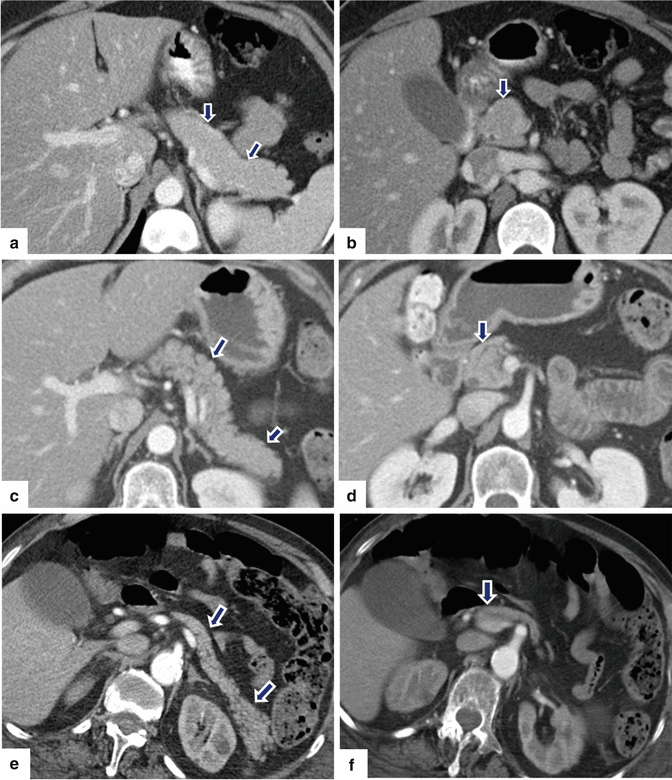
Fig. 5.21
Normal age-related changes of the pancreas on contrast-enhanced CT. (a, b) Axial images of the pancreas of a 30-year-old female show a pancreas with a homogeneous density and smooth margins (arrows). (c, d) Axial images of the pancreas of a 59-year-old female show a pancreas with a heterogeneous density and lobulated margins (arrows). (e, f) Axial images of the pancreas of an 86-year-old female show an atrophic pancreas with heterogeneous density and slightly lobulated margins (arrows)
The appearance of the pancreas on CT depends on the amount of fat within the intralobular septa that separates the acinar lobules of the gland.
The margins of the pancreas are smooth in younger patients.
The density of the parenchyma is homogeneous, with an attenuation similar to that of the muscle and spleen but less than non-contrast studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree