For pathologic conditions affecting the skull base and cerebellopontine angle, imaging techniques have advanced to assess for residual disease, disease progression, and postoperative complications. Knowledge regarding various surgical approaches of skull base tumor resection, expected postoperative appearance, and common postsurgical complications guides radiologic interpretation. Complexity of skull base anatomy, small size of the relevant structures, lack of familiarity with surgical techniques, and postsurgical changes confound radiologic evaluation. This article discusses the imaging techniques, surgical approaches, expected postoperative changes, and complications after surgery of the skull base, with emphasis on the cerebellopontine angle, anterior cranial fossa, and central skull base regions.
Key points
- •
Knowledge regarding various approaches of skull base surgery, expected postoperative appearance, and common postsurgical complications can guide radiologic interpretation.
- •
Although there are predictable imaging changes following surgery, it is difficult to differentiate treatment change and residual disease at a single time point and immediate postsurgical imaging and follow-up imaging are often complementary.
- •
CSF leak is a common complication of skull base surgery. Other complications shared by multiple types of surgical approaches include infarct, vascular injury/thrombosis, and infection.
Introduction
For many pathologic conditions affecting the skull base and cerebellopontine angle (CPA), surgery is part of the treatment process. As surgical techniques and approaches have evolved through the years, imaging techniques have also advanced in a parallel manner to assess for residual disease, disease progression, and postoperative complications. Complexity of skull base anatomy, small size of the relevant structures, lack of familiarity with surgical techniques, and postsurgical changes can confound radiologic evaluation. This article discusses the imaging techniques, surgical approaches, expected postoperative changes, and complications after surgery of the skull base, with emphasis on the CPA, anterior cranial fossa, and central skull base regions.
Imaging techniques
Computed tomography (CT) and MR imaging are mainstays in the imaging of the skull base and CPA. Modern multislice CT scanners can acquire images as thin as 0.5 mm with multiplanar evaluation in axial, coronal, sagittal, and oblique planes. CT covering the mastoid bones, temporal bones, and skull base without the administration of intravenous contrast is performed in postoperative assessment, primarily for evaluation of the bony integrity of the reconstructed skull base. In particular, CT can evaluate for osseous defects in cases of suspected cerebrospinal fluid (CSF) leak. In cases where site of leak remains uncertain, CT imaging following injection of intrathecal contrast (CT cisternography) can aid in detection of subtle CSF leak, evidenced by abnormal accumulation of hyperattenuating CSF in the suspected anatomic compartment. CT angiography may be used in the emergency setting for assessment of intraoperative vascular injury or thrombosis, performed with bolus contrast injection in arterial and/or venous phase. Evaluation of the vasculature is enhanced with multiplanar maximum intensity projection and three-dimensional (3D) rotational reconstructions. When time is of the essence, conventional catheter angiography may be initiated instead of CT angiography for diagnosis and treatment purposes, including embolization or vessel sacrifice.
MR imaging is superior to CT for the evaluation of soft tissue lesions, with contrast-enhanced CT an alternative for patients with contraindications to MR imaging. Whenever possible, this is done with higher magnetic strength at 3 T for improved soft tissue discrimination. In addition to routine brain imaging, small field of view T1 precontrast and fat-suppressed T1 postcontrast sequences are obtained centered on the region of interest, be it the sella/central skull base, temporal bones, or orbit/face. Dedicated T2 images without and/or with fat suppression are also helpful in the postoperative analysis.
High-resolution precontrast and postcontrast T1 and T2 3D MR imaging of the skull base are especially helpful in identifying anatomic structures of the skull base because they provide excellent signal to noise ratio and high spatial resolution. , Typical 3D protocol involves precontrast and postcontrast T1 and T2 image acquisition with a slice thickness of 0.6 mm, matrix of 256 × 256, and field of view of 16.9 × 24.6. 3D volumetric acquisition of the skull base is performed to encompass the region of interest, with subsequent creation of isotopic multiplanar reconstructions. Heavily T2-weighted images, such as constructive interference in steady-state (CISS, Siemens) or FIESTA (GE), provide excellent anatomic detail with respect to structures of interest and surrounding CSF fluid. 3D T1-weighted, non–fat saturated images before the administration of contrast allows for evaluation of intrinsic T1 signal. Postcontrast T1 (MPRAGE or VIBE) and T2 images can help distinguish vessels, nerves, and pathologic tissue enhancement. Heavy T2 weighting also allows for detection of CSF with delineation of small skull base defects and areas of CSF leakage. , A 3D T2-weighted sequence with fat saturation, such as short tau inversion recovery, has the benefit for detecting intrinsic T2 signal abnormalities.
Steady-state free precession, such as CISS, is limited by banding artifacts that present as linear bands of low signal that are most evident at the air-tissue interfaces caused by field inhomogeneities. Banding artifacts are ameliorated by alternating the field of the radiofrequency pulse by 180° and by keeping TR as low as possible.
Surgical techniques
Cerebellopontine Angle
Vestibular schwannomas are the most common intracranial tumor involving the CPA cistern, accounting for approximately 80% of all tumors in this location. Meningiomas and epidermoids account for a smaller percentage of tumors in the CPA, making up approximately 10% and 6%, respectively. Other tumors of the CPA can include petroclival masses, such as chondrosarcoma, endolymphatic sac tumors, and paragangliomas. Excision of these lesions is the treatment of choice, by translabyrinthine, retrosigmoid (suboccipital), and middle cranial fossa approaches depending on the tumor size, location, patient’s hearing status, and preference of the operating surgeon. Similar approaches are considered with debulking of infectious or inflammatory diseases in these locations. In patients with trigeminal neuralgia, the retrosigmoid approach is used for microvascular decompression of the trigeminal nerve.
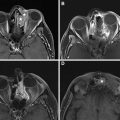
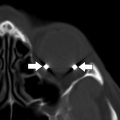
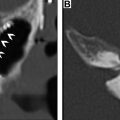
Translabyrinthine resection is preferred when the lesion is within the labyrinthine and cochlear structures, and in cases of larger CPA tumors. This approach involves a total mastoidectomy and labyrinthectomy with resection of the semicircular canals and portions of the vestibule with placement of a triangular fat graft over the mastoidectomy bed ( Fig. 1 ). Hearing ability is often sacrificed during the surgery so this approach may also be used in cases of existing pronounced ipsilateral hearing loss. The ossicles are sometimes removed and packing material, such as fat, fascia, or fibrin, is placed in the middle ear to limit occurrence of CSF leak. ,

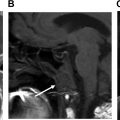
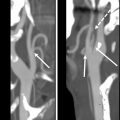
The retrosigmoid/suboccipital approach involves craniotomy or craniectomy with cranioplasty and dural incision from the superior margin of the transverse sinus to the sigmoid sinus ventrally ( Fig. 2 ). The posterior mastoid air cells are often violated, but are promptly sealed with bone wax to prevent CSF leak. The posterior wall of the internal auditory canal (IAC) may be resected with or without internal labyrinthectomy to access the IAC fundus. In the setting of trigeminal neuralgia, the retrosigmoid approach facilitates microvascular decompression of the trigeminal nerve in which a piece of shredded Teflon is placed between the offending vessel and the nerve ( Fig. 3 ).


A temporal craniotomy is performed for the middle cranial fossa approach resection with dissection of the dura over the petrous ridge and middle cranial fossa. The temporal lobe is then carefully retracted superiorly with a narrow exposure of the CPA cistern to limit the risk of temporal lobe injury. , Bone around the IAC is removed and the roof of the IAC is exposed.
Anterior Craniofacial Resection
Most malignant tumors of the anterior skull base including squamous cell carcinoma, sinonasal undifferentiated carcinoma, adenocarcinoma, and esthesioneuroblastoma arise from the sinonasal cavity. The anterior skull base may also be involved through local invasion from tumors of the face, scalp, or orbits. Malignant tumors of the paranasal sinuses and nasal cavity account for 3% of head and neck tumors, with tumors of the ethmoid sinuses comprising 20% to 30% of paranasal sinus malignancies. Surgery remains the gold standard for treatment. Ethmoid tumors invariably encroach on the cribriform plate, and anterior craniofacial resection is usually required for complete tumor resection. , This approach can also be taken for skull base revision following extensive craniofacial trauma or for surgical debridement of complex infections, such as in cases of acute sinusitis complicated by anterior frontal osteomyelitis and subperiosteal abscess (Pott puffy tumor).
Anterior craniofacial resection involves a combination of transcranial and transfacial techniques and is often combined with endonasal endoscopic approaches. The transcranial component is typically initiated with a bicoronal incision and low frontal craniotomy. The transfacial approach involves a lateral rhinotomy incision that is extended into a radical maxillectomy if more extensive maxillary resection is necessary. Orbital exenteration may be performed if there is orbital tumor infiltration on preoperative imaging and vision is not salvageable. The anterior craniofacial technique allows exposure of the epidural space, sinonasal cavity, and anterior cranial fossa, allowing en bloc radical resection and has evolved to be the standard of care in anterior skull base malignancies. In cases with dural or intradural involvement, the tumor is resected with microsurgical technique with placement of a dural patch graft. After resection, the floor of the anterior cranial fossa is reconstructed using a graft, such as metallic mesh, pedicled pericranial flap, osseous graft, or free flap. , , In cases of extensive trauma, tumor, or infection involving the frontal sinuses, the frontal sinuses are often cranialized during surgery. This involves resection of the posterior wall of the frontal sinuses with removal of the sinus mucosa so that the frontal lobe dura lies adjacent to the floor and outer table of the frontal sinus, often accompanied by pericranial flap placement ( Fig. 4 ).

Transsphenoidal and Pterional Surgery
Endoscopic endonasal transsphenoidal approach surgery ( Fig. 5 ) is a minimally invasive technique for the resection of sellar and parasellar tumors, and has been the standard surgical treatment of pituitary adenomas for decades. The technique can also be modified for the resection of other pathologies, such as anterior cranial fossa meningiomas and clival chordomas, with expanded transcribiform, transplanum, transsellar, and transclival approaches to gain a surgical window into the anterior cranial fossa, suprasellar space, or clivus. ,

There are several traditional endonasal approaches that are considered for sellar surgery, including paraseptal, middle turbinectomy, and middle meatal approaches. In the paraseptal approach, a surgical window is created by fracturing and displacing the nasal septum and middle turbinate laterally from the ventral wall of the sphenoid sinus. The middle turbinectomy approach involves resection of the lower middle turbinate to open a narrowed nasal cavity. A middle meatal approach may be taken if there is tumor predominantly in the lateral sella or cavernous sinus with the surgical corridor between the middle turbinate and lateral nasal cavity wall.
Once the rostrum of the sphenoid sinus is exposed, anterior sphenoidotomy is performed. A sphenoid septectomy is performed with osseous fragments saved for later sellar reconstruction. The anterior sella wall is then located and opened. After tumor resection is completed, microdissective resection is performed at the interface of the tumor with normal pituitary tissue. A fat graft is then harvested from the periumbilical region and is placed in the resection bed in the situation of a large resection cavity or if intraoperative CSF leak is suspected. The anterior wall of the sella is then reconstructed using the previous saved fragments of bone. The anterior sellar reconstruction is supported with packing of the sphenoid sinuses with absorbable gelatin sponge. Other sealant materials include titanium mesh and DuraGen. , A vascular pedicle nasal septal mucoperiosteal flap covering the defect has helped decrease the incidence of CSF leaks in recent years. The flap is placed over fat or directly onto the dura, with graft fixation using a biologic glue, and nasal packing or a Foley balloon holding the balloon in place.
Similar to clipping of anterior circulation aneurysms, a pterional approach resection is sometimes used for removal or biopsy of suprasellar and parasellar lesions. After frontotemporal craniotomy is accomplished, retraction of the frontal and temporal lobes is preceded by opening of the anterior limb of the sylvian fissure, reducing retraction needed for adequate exposure. The arachnoid layers over the ipsilateral optic nerve and carotid artery dissected and the contralateral neurovascular structure are identified and pathologic tissue is carefully dissected away from these structures.
Expected postoperative imaging findings
General expected postsurgical changes after skull base tumor resection include scalp edema and subcutaneous emphysema, manifest as high T2 signal over the craniotomy site with scattered foci of susceptibility on MR imaging. Diffusion restriction along the margins of the surgical tract is seen, representing postoperative cytotoxic edema. Heterogeneous intensity material is visualized in the resection cavity, which can represent a combination of postsurgical hemorrhage, air, and packing material. T2 hyperintense extra-axial fluid collections are seen subjacent to the craniotomy site or over the brain convexities.
Cerebellopontine Angle
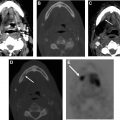
In the CPA, there is a characteristic flattened appearance of the lateral margin of the cerebellar hemisphere with prominence of the adjacent T2 hyperintense extra-axial CSF space as a result of retrosigmoid entry and cerebellar retraction ( Fig. 6 ). , Linear enhancement in the surgical bed on early postoperative imaging is almost always present, likely reflecting a combination of granulation tissue and reactive dural thickening. Nodular enhancement is suspicious for residual tumor especially with subsequent enlargement on follow-up imaging, whereas postoperative reactive enhancement remains stable or decreases over time ( Fig. 7 ). , , For vestibular schwannoma resection via retrosigmoid approach, the IAC fundus is a frequent blind spot and therefore this area should be scrutinized for residual tumor. Residual tumor may sometimes be intentionally left behind for functional preservation of the facial nerve. ,