Masses in the sella and parasellar region comprise about 10% of all pediatric brain tumors but type and frequency differs from those in adults. Imaging is critical for diagnosis and characterization of these lesions. By assessing the site of origin, signal and contrast enhancement characteristics, and the presence or absence of characteristic patterns, differential diagnosis can narrow the possibilities. The clinical presentation is often characteristic for lesion type and should be considered. This article summarizes the characteristic imaging features of the most frequent pediatric tumors and tumor-mimicking lesions in children in this region.
Key points
- •
Sellar and parasellar masses are not infrequent in the pediatric population, comprising about 10% of all pediatric brain tumors.
- •
Key features that help to distinguish between these lesions include the primary site of origin (eg, sellar, suprasellar, infundibular), the intrinsic signal and enhancement pattern, and the age and clinical presentation of the patient.
- •
There are many important differences between children and adults with respect to the type and frequency of sellar and suprasellar masses.
Introduction
Masses arising in the sella and parasellar region are not infrequent in the pediatric population, comprising about 10% of all pediatric brain tumors. Imaging, especially Magnetic resonance (MR) imaging, which renders high soft tissue contrast and great anatomic detail, remains a critical component in diagnosis and characterization of these lesions, helping suggest whether lesions are most likely benign or malignant.
Some of the key features on MR imaging that allow one to distinguish 1 type of lesion from another in the sellar and suprasellar regions include primary location and extension, the intrinsic signal and contrast enhancement patterns, and the presence or absence of distinguishing features, such as cysts and calcifications. Based on location, lesions are classified as either entirely intrasellar, sellar and suprasellar, infundibular, or entirely suprasellar. Lesions can be further characterized as being entirely solid, entirely cystic, or partially solid and partially cystic. This article summarizes the characteristic imaging features of the most frequent pediatric tumors and tumor-mimicking lesions in this region.
Historically, intrasellar lesions were indirectly imaged or diagnosed with plain skull radiographs and polytomography based on widening of the sella and the presence of calcifications in the sella and suprasellar region. Pneumoencephalography was used to outline sellar and suprasellar tumors. Currently, MR imaging is the primary imaging modality for sellar and suprasellar lesions, especially in the pediatric age group. Computed tomography (CT) may occasionally provide complementary information when more definitive assessment for the presence of calcifications is desired. Magnetic resonance (MR) imaging of the pituitary and suprasellar region is performed on a 1.5 T or 3 T MR scanner with conventional T1-weighted and T2-weighted, multiplanar, thin-slice, noncontrast, and gadolinium-enhanced MR sequences. In general, sagittal and coronal planes are most advantageous in evaluating this region. Additional sequences may be added in specific situations, such as when hemorrhage (susceptibility weighted imaging [SWI]) or a microadenoma (dynamic contrast-enhanced imaging) is suspected. High-resolution T2-weighted isotropic sequences may also occasionally be helpful when exact anatomic relationships need to be assessed, such as when trying to determine if a cystic mass arises from the third ventricle or is extending into it or where important functional structures, such as the optic chiasm, are located in relation to the lesion. The standard pituitary protocol performed at the corresponding author’s institution listed in Table 1 . Finally, advanced imaging sequences such as diffusion-weighted imaging (DWI), diffusion tensor imaging, perfusion-weighted imaging, or hydrogen proton ( 1 H) MR spectroscopy are progressively used to further narrow down the differential diagnosis.
| Plane | Sequence Name |
|---|---|
| Sagittal | T1 3D sagittal |
| Axial | T2 fluid-attenuated inversion recovery (FLAIR) axial |
| Axial | T2 axial (no fat saturation) |
| Axial | Diffusion tensor imaging |
| Coronal | T1 coronal thin (pituitary) |
| Sagittal | T1 sagittal thin (pituitary) |
| Contrast bolus 1× dose (0.1 mmol/kg): 1 cc/sec, dynamic pituitary if ordered | |
| Coronal | T1 postcoronal thin (pituitary) |
| Sagittal | T1 postsagittal thin (pituitary) |
| Axial | T1 axial post |
In addition to the imaging findings, the clinical presentation can offer important clues regarding the diagnosis and primary location of the tumor. Depending on the size and location of the lesion, the clinical presentation can vary from nonspecific symptoms caused by increased intracranial pressure to more specific symptoms related to pituitary hormone deficiencies or excesses. Vision changes are also not uncommon and occur secondary to mass effect on the optic apparatus. Some lesions are asymptomatic and are incidentally found during imaging of the brain for other indications.
There are many differences between children and adults with respect to sellar and suprasellar tumors. For example, the type and frequency of tumors seen is quite different. Thus, macroadenomas are common in adults but rare in children. Similarly, optic gliomas are common childhood tumors but do not typically arise de novo in adults. There are also different challenges when it comes to imaging the pediatric sellar and suprasellar region. Compared with adults, pediatric imaging requires a smaller field of view and thinner slices because of the smaller anatomic structures involved. Finally, the response to increased intracranial pressure is different in young children compared with adults. The presence of open sutures allows sutural diastasis in the setting of increasing intracranial pressure, sometimes accommodating very large intracranial masses before the patient becomes symptomatic.
Normal Anatomy and Pituitary Embryology
Identification of abnormalities in the sellar and parasellar regions requires a basic familiarity with both the normal anatomy and embryologic development of the pituitary gland. The pituitary gland is composed of 2 different parts with embryonically distinct development: the anteriorly located adenohypophysis and the posteriorly located neurohypophysis. The adenohypophysis is thought to derive from Rathke pouch, which separates during embryologic development from the oral ectoderm, then migrates upwards toward the infundibulum, a downward extension from the hypothalamus. The infundibulum represents the budding neurohypophysis that eventually extends inferiorly to the sella to form the posterior pituitary proper. The adenohypophysis is made up of 3 parts: the pars tuberalis, the pars intermedia, and the pars distalis. The pars tuberalis is located in the pituitary stalk where it surrounds the neurohypophyseal axons extending from the hypothalamus to the posterior pituitary proper. The pars distalis is the largest, most anterior portion of the adenohypophysis, and the pars intermedia is the portion located directly in contact with the neurohypophysis.
In both preterm and term newborns, the anterior lobe of the pituitary (adenohypophysis) is characteristically T1 hyperintense, and gradually becomes T1 isointense to the pons by 6 to 8 weeks of life. The normal craniocaudal dimension in children younger than 12 years of age is less than 6 mm and the upper surface should be flat or slightly concave. In pregnancy, during lactation, and during puberty, the upper margin can become convex superiorly and pituitary heights up to 12 mm can be normal. In men and postmenopausal women, the normal pituitary craniocaudal dimension is less than 8 mm and, in young menstruating girls and women, less than 10 mm. The anterior pituitary takes up about 70% of the volume of the gland and has a homogenous appearance on both precontrast and postcontrast sequences. The posterior pituitary has a characteristic intrinsic T1 hyperintensity, which is also known as the posterior pituitary bright spot. The infundibulum (or pituitary stalk) is widest at its origin at the hypothalamus and tapers to its distal attachment at the level of the pituitary gland. Both the adenohypophysis and neurohypophysis, including pituitary stalk, show strong contrast enhancement. At the insertion of the pituitary stalk, a focal hyperenhancement may be seen that matches the distal component of the portal venous system that connects the adenohypophysis with the hypothalamus.
Introduction
Masses arising in the sella and parasellar region are not infrequent in the pediatric population, comprising about 10% of all pediatric brain tumors. Imaging, especially Magnetic resonance (MR) imaging, which renders high soft tissue contrast and great anatomic detail, remains a critical component in diagnosis and characterization of these lesions, helping suggest whether lesions are most likely benign or malignant.
Some of the key features on MR imaging that allow one to distinguish 1 type of lesion from another in the sellar and suprasellar regions include primary location and extension, the intrinsic signal and contrast enhancement patterns, and the presence or absence of distinguishing features, such as cysts and calcifications. Based on location, lesions are classified as either entirely intrasellar, sellar and suprasellar, infundibular, or entirely suprasellar. Lesions can be further characterized as being entirely solid, entirely cystic, or partially solid and partially cystic. This article summarizes the characteristic imaging features of the most frequent pediatric tumors and tumor-mimicking lesions in this region.
Historically, intrasellar lesions were indirectly imaged or diagnosed with plain skull radiographs and polytomography based on widening of the sella and the presence of calcifications in the sella and suprasellar region. Pneumoencephalography was used to outline sellar and suprasellar tumors. Currently, MR imaging is the primary imaging modality for sellar and suprasellar lesions, especially in the pediatric age group. Computed tomography (CT) may occasionally provide complementary information when more definitive assessment for the presence of calcifications is desired. Magnetic resonance (MR) imaging of the pituitary and suprasellar region is performed on a 1.5 T or 3 T MR scanner with conventional T1-weighted and T2-weighted, multiplanar, thin-slice, noncontrast, and gadolinium-enhanced MR sequences. In general, sagittal and coronal planes are most advantageous in evaluating this region. Additional sequences may be added in specific situations, such as when hemorrhage (susceptibility weighted imaging [SWI]) or a microadenoma (dynamic contrast-enhanced imaging) is suspected. High-resolution T2-weighted isotropic sequences may also occasionally be helpful when exact anatomic relationships need to be assessed, such as when trying to determine if a cystic mass arises from the third ventricle or is extending into it or where important functional structures, such as the optic chiasm, are located in relation to the lesion. The standard pituitary protocol performed at the corresponding author’s institution listed in Table 1 . Finally, advanced imaging sequences such as diffusion-weighted imaging (DWI), diffusion tensor imaging, perfusion-weighted imaging, or hydrogen proton ( 1 H) MR spectroscopy are progressively used to further narrow down the differential diagnosis.
| Plane | Sequence Name |
|---|---|
| Sagittal | T1 3D sagittal |
| Axial | T2 fluid-attenuated inversion recovery (FLAIR) axial |
| Axial | T2 axial (no fat saturation) |
| Axial | Diffusion tensor imaging |
| Coronal | T1 coronal thin (pituitary) |
| Sagittal | T1 sagittal thin (pituitary) |
| Contrast bolus 1× dose (0.1 mmol/kg): 1 cc/sec, dynamic pituitary if ordered | |
| Coronal | T1 postcoronal thin (pituitary) |
| Sagittal | T1 postsagittal thin (pituitary) |
| Axial | T1 axial post |
In addition to the imaging findings, the clinical presentation can offer important clues regarding the diagnosis and primary location of the tumor. Depending on the size and location of the lesion, the clinical presentation can vary from nonspecific symptoms caused by increased intracranial pressure to more specific symptoms related to pituitary hormone deficiencies or excesses. Vision changes are also not uncommon and occur secondary to mass effect on the optic apparatus. Some lesions are asymptomatic and are incidentally found during imaging of the brain for other indications.
There are many differences between children and adults with respect to sellar and suprasellar tumors. For example, the type and frequency of tumors seen is quite different. Thus, macroadenomas are common in adults but rare in children. Similarly, optic gliomas are common childhood tumors but do not typically arise de novo in adults. There are also different challenges when it comes to imaging the pediatric sellar and suprasellar region. Compared with adults, pediatric imaging requires a smaller field of view and thinner slices because of the smaller anatomic structures involved. Finally, the response to increased intracranial pressure is different in young children compared with adults. The presence of open sutures allows sutural diastasis in the setting of increasing intracranial pressure, sometimes accommodating very large intracranial masses before the patient becomes symptomatic.
Normal Anatomy and Pituitary Embryology
Identification of abnormalities in the sellar and parasellar regions requires a basic familiarity with both the normal anatomy and embryologic development of the pituitary gland. The pituitary gland is composed of 2 different parts with embryonically distinct development: the anteriorly located adenohypophysis and the posteriorly located neurohypophysis. The adenohypophysis is thought to derive from Rathke pouch, which separates during embryologic development from the oral ectoderm, then migrates upwards toward the infundibulum, a downward extension from the hypothalamus. The infundibulum represents the budding neurohypophysis that eventually extends inferiorly to the sella to form the posterior pituitary proper. The adenohypophysis is made up of 3 parts: the pars tuberalis, the pars intermedia, and the pars distalis. The pars tuberalis is located in the pituitary stalk where it surrounds the neurohypophyseal axons extending from the hypothalamus to the posterior pituitary proper. The pars distalis is the largest, most anterior portion of the adenohypophysis, and the pars intermedia is the portion located directly in contact with the neurohypophysis.
In both preterm and term newborns, the anterior lobe of the pituitary (adenohypophysis) is characteristically T1 hyperintense, and gradually becomes T1 isointense to the pons by 6 to 8 weeks of life. The normal craniocaudal dimension in children younger than 12 years of age is less than 6 mm and the upper surface should be flat or slightly concave. In pregnancy, during lactation, and during puberty, the upper margin can become convex superiorly and pituitary heights up to 12 mm can be normal. In men and postmenopausal women, the normal pituitary craniocaudal dimension is less than 8 mm and, in young menstruating girls and women, less than 10 mm. The anterior pituitary takes up about 70% of the volume of the gland and has a homogenous appearance on both precontrast and postcontrast sequences. The posterior pituitary has a characteristic intrinsic T1 hyperintensity, which is also known as the posterior pituitary bright spot. The infundibulum (or pituitary stalk) is widest at its origin at the hypothalamus and tapers to its distal attachment at the level of the pituitary gland. Both the adenohypophysis and neurohypophysis, including pituitary stalk, show strong contrast enhancement. At the insertion of the pituitary stalk, a focal hyperenhancement may be seen that matches the distal component of the portal venous system that connects the adenohypophysis with the hypothalamus.
Intrasellar lesions
Rathke Cleft Cyst
Rathke cleft cysts are benign cysts arising from remnants of Rathke cleft (lumen between anterior and posterior walls of Rathke pouch) that fails to involute during development. The walls of the cysts are lined by columnar or cuboidal epithelium. They are not uncommon in the general population and found in about 4% of routine autopsies. Compared with the adult population, they are less common and smaller in size in children, typically measuring less than 2 mm in children younger than 9 years of age, likely due to their natural history of slow expansion over time. They are often asymptomatic and found incidentally, especially when small. When larger, they may cause symptoms from mass effect, including headache, visual symptoms, and pituitary dysfunction. They may rarely bleed and cause pituitary apoplexy-like symptoms.
On imaging, Rathke cleft cysts are centered between the anterior and posterior lobes of the pituitary gland and are either entirely intrasellar; intrasellar with suprasellar extension; and, uncommonly, entirely suprasellar ( Fig. 1 ). With suprasellar extension, they are located along the anterior aspect of the infundibulum. Their T1 signal intensity varies from hypointense (serous cystic contents) to more hyperintense with increasing proteinaceous or mucoid contents of the cyst. There is no solid enhancing tissue and the walls of the cyst do not enhance but there may be pseudoenhancement of the walls when enhancing pituitary gland is stretched over the cyst walls (claw sign). A characteristic nonenhancing T1 hyperintense nodule is present in 44% to 77% of Rathke cleft cysts.
Features differentiating Rathke cleft cysts from other cystic pituitary lesions, in particular craniopharyngiomas (CPs) and cystic adenomas, include its sharply demarcated appearance with homogenous signal intensity, lack of a solidly enhancing component, and characteristic location between the anterior and posterior lobes of the pituitary. In the setting of a CP, normal pituitary gland can often be identified inferior to the tumor, and there is often calcification as well as tumor wall enhancement. Pituitary adenomas are centered within the adenohypophysis and cystic components have thick, irregular, enhancing walls. Finally, the cytokeratin expression pattern from pathologic specimens can differentiate Rathke cleft cysts from CPs because the former express cytokeratins 8 and 20, whereas the latter do not.
In young children, small cysts (<3 mm) are sometimes seen incidentally in the pars intermedia region and often referred to as pars intermedia cysts. These are also derived from embryologic remnants of the Rathke cleft and do not require follow-up imaging in asymptomatic individuals.
Craniopharyngioma
CPs are the most common pediatric intracranial tumor of nonglial origin. They account for about 5% of intracranial tumors in children and up to 50% of pediatric suprasellar tumors. There are 2 main histologic subtypes: the adamantinomatous and the squamous-papillary. Adamantinomatous CP has a bimodal age distribution, with most cases arising in the pediatric age group and a smaller peak in older adults (>65 years), whereas papillary CP occurs mostly in adults older than 50 years of age. The prevailing theory for pathogenesis of CPs is the embryogenetic theory, whereby adamantinomatous CPs arise from epithelial remnants of Rathke pouch along the craniopharyngeal duct, which is the pathway of migration of Rathke pouch from the stomodeum (embryologic precursor of the mouth) to the infundibulum. The squamous papillary subtype is thought to occur by squamous metaplasia of cells derived from the pars tuberalis of the adenohypophysis. Clinical symptoms arise from either local mass effect or increased intracranial pressure, and include headache, visual disturbance, and endocrine abnormalities, including decreased growth rate and polydipsia or polyuria.
CPs are most commonly both intrasellar and suprasellar in location (53%–75%), followed by purely suprasellar (20%–41%), and least commonly purely intrasellar (5%–6%). Although characteristic imaging features have been described for adamantinomatous and squamous-papillary CP subtypes, some investigators maintain that there are no reliable distinguishing features between them. In general, adamantinomatous CPs are described as lobulated multicystic tumors with enhancing walls and calcifications, typically with a solid enhancing portion also present ( Fig. 2 ). The cyst contents are T1 hyperintense in about 33% of cases due to high protein content or methemoglobin. The squamous-papillary subtype, in contrast, is described as more spherical and predominantly solid. If cystic components are present, they are typically T1 hypointense and calcifications are atypical. This subtype can sometimes arise in ectopic locations, such as the floor of the third ventricle, the posterior fossa, or the nasopharynx. For presurgical planning, CPs can be classified into groups based on their relationship to the optic chiasm as either sellar (sellar ± suprasellar extension without mass effect on the chiasm), prechiasmatic (centered anterior to the optic chiasm with posterior or superior displacement of the optic chiasm), or retrochiasmatic (centered posteriorly with anterior displacement of the optic chiasm). In addition, their relationship to the hypothalamus, third ventricle, and vessels of the circle of Willis is important to document in the report.
Although these tumors are benign, malignant transformation can rarely occur after irradiation. Rarely, CPs may spread along the surgical path or more distantly through leptomeningeal spread. Adamantinomatous CPs are histologically benign but biologically aggressive and have a tendency to invade surrounding structures, which often prevents gross total resection. For squamous-papillary CPs, gross total resection is typically curative.
Pituitary Adenoma
Although common in the adult population, pituitary adenomas are rare in children, and comprise less than 3% of supratentorial tumors in this age group. On the other hand, pituitary adenomas are quite common in the setting of a purely intrasellar enhancing lesion, so should probably be considered as a diagnostic possibility, even in the pediatric age group. In children, most are of the functional variety, with prolactinomas accounting for about half of those, followed by corticotropin-secreting tumors and growth hormone–secreting tumors. Prolactinomas are seen most commonly in female patients and tend to present with galactorrhea and amenorrhea. Nonfunctioning adenomas tend to present when they are larger and produce mass effect on adjacent structures, which can result in headache and visual disturbances.
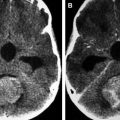
Imaging appearance of microadenomas (<10 mm in size) and macroadenomas (>10 mm in size) is identical to that of the adult population. Prolactin and growth hormone–secreting microadenomas tend to be located more laterally in the adenohypophysis, whereas corticotropin, thyroid-stimulating hormone, and gonadotropin-secreting microadenomas tend to be located more medially. Microadenomas often show delayed enhancement relative to the normal adjacent pituitary gland, with peak enhancement occurring after about 1 to 4 minutes. This differential enhancement pattern can be taken advantage of in detection of otherwise difficult to identify microadenomas by using thin section coronal T1-weighted dynamic precontrast and postcontrast imaging. On unenhanced sequences, microadenomas are typically isointense or hypointense on T1-weighted images compared with the normal surrounding gland ( Fig. 3 ). On T2-weighted imaging, their appearance is more variable but tends to be bright (80% of prolactinomas) and those tend to be softer tumors that are easier to resect than those that are T2 dark. Macroadenomas often extend beyond the confines of the pituitary gland, most commonly into the suprasellar cistern (80%), with less common extension into the cavernous sinus, sphenoid sinus, or dorsum sella (see Fig. 3 ). Their signal is often more heterogeneous than that of microadenomas and is related to cystic, necrotic, and hemorrhagic components (see Fig. 3 ). If possible, it is important to try to identify any normal-appearing remaining pituitary tissue because its preservation is the goal during surgery to prevent pituitary insufficiency.
Pituitary Hyperplasia
Pituitary hyperplasia is defined as a nonneoplastic polyclonal absolute increase in the number of 1 or more adenohypophyseal cell subtypes, which results in enlargement of the gland. This may be physiologic as, for example, during puberty, pregnancy, or during lactation. Pathologic pituitary hyperplasia, on the other hand, is present when the gland enlarges beyond what is considered normal as predicted by the patient’s age, gender, and physiologic state. In pregnancy and in the postpartum state, for example, it is normal to see physiologic enlargement of the pituitary gland height to up to approximately 12 mm. Pathologic pituitary hyperplasia is most commonly encountered in the setting of end-organ deficiency, including thyroid insufficiency and primary hypogonadism. Thyroid insufficiency and lack of thyroxine hormone results in overproduction of hypothalamic thyrotropin-releasing hormone, which in turn leads to thyrotroph hyperplasia in the pituitary gland and increased thyroid-stimulating hormone production. In addition, prolactin releasing cells may be stimulated and these patients may present clinically due to symptoms related to hyperprolactinemia.
On imaging, the normal pituitary gland in children should be less than 6 mm in height and in young menstruating female patients less than 10 mm in height. During puberty, and in pregnant as well as lactating patients, pituitary heights up to 12 mm can be normal ( Fig. 4 ). In both physiologic and pathologic pituitary hyperplasia, the pituitary gland demonstrates diffuse symmetric enlargement and, especially in pathologic cases, the gland can extend far into the suprasellar cistern, even causing mass effect on the prechiasmatic optic nerves and optic chiasm. In contrast to macroadenomas, there is no remodeling of the sella, and the gland will always show homogenous postcontrast enhancement. On precontrast and postcontrast T1-weighted images, as well as on T2-weighted images, the gland will be homogenous in signal (see Fig. 4 ).
Empty Sella
The empty sella, defined as a pituitary gland measuring 2 mm or less in height with cerebrospinal fluid (CSF) occupying greater than half of the sella (see Fig. 4 ), is rare in children, with an incidence of about 1% in patients with a normal hypothalamic-pituitary axis. Primary empty sella occurs when CSF enters the sella through a rent in the sellar diaphragm and may or may not be associated with increased intracranial pressure. Secondary empty sella occurs as a result of injury to the pituitary itself, for example, in the setting of pituitary apoplexy, or following surgery or radiation treatment. In adults, the empty sella is commonly seen in older, obese, or hypertensive patients, and may often be asymptomatic. In children, conversely, an empty sella is more likely associated with clinical symptoms and endocrinopathies, especially growth hormone deficiency, hypogonadism, or multiple pituitary hormone deficiency. In children with known endocrinopathy, empty sella may be seen in up to 68% of patients. Thus, the presence of an empty sella in the pediatric population should prompt endocrinologic and ophthalmologic evaluation.
Infundibular lesions
Pediatric patients with central diabetes insipidus typically have absence of the posterior pituitary T1-bright spot and about one-third of them also show thickening of the infundibulum. The most common causes in these cases are Langerhans cell histiocytosis (LCH) and germinoma (see later discussion). If the infundibulum is initially normal on imaging, follow-up imaging in these patients with so-called idiopathic central diabetes insipidus is warranted because a time lag of up to 14 months has been reported in detecting an infundibular lesion. Other differential considerations for infundibular lesions in the pediatric age group include granulomatous disease (eg, sarcoid and tuberculosis [TB]) and lymphocytic hypophysitis.
Langerhans Cell Histiocytosis
LCH is a rare idiopathic disorder characterized by proliferation of Langerhans cell histiocytes, resulting in the formation of granulomas and can be found in any organ system. The clinical course of LCH is variable, ranging from a solitary lytic bone or skin lesions with complete remission to a multisystem disorder with potentially lethal outcome. The peak incidence is between the ages of 1 to 4 years but LCH can present at any age from the newborn period to the geriatric age group. Intracranial involvement most commonly occurs in the form of a lytic bone mass or diabetes insipidus. In multicentric LCH, the hypothalamus and infundibulum are involved in up to 20% of patients and, in those cases, diabetes insipidus and absence of the posterior pituitary bright spot are also typically present. When involved, the infundibulum becomes focally or diffusely thickened (typically > 3.5 mm if proximal, or >2 mm if distal) and enhances avidly. The pituitary gland itself may also be involved in up to about 10% of patients with central nervous system (CNS) involvement, with imaging demonstrating infiltration and enlargement of the gland.
Neither absence of the posterior pituitary bright spot nor thickening of the infundibulum is specific to LCH, and there is significant imaging overlap with germinomas and the other entities discussed in this section ( Fig. 5 ). However, there may often be additional findings to suggest the diagnosis of LCH. For example, calvarial involvement with lytic punched-out bone lesions in the skull, including the frontal, parietal, and mastoid portion of the temporal bones, is common, as are involvement of the orbit and facial bones. Additional findings that may be encountered include prominent Virchow-Robin spaces, white matter parenchymal changes with a leukoencephalopathy-like pattern, and gray matter signal changes in the cerebellar dentate nucleus and basal ganglia.