Skeletal muscle tumors
Benign
Extracardiac rhabdomyoma : adult, fetal, and genital type
Malignant
Embryonal rhabdomyosarcoma
Alveolar rhabdomyosarcoma
Pleomorphic rhabdomyosarcoma
Spindle cell/sclerosing rhabdomyosarcoma
Smooth muscle tumors
Benign
Deep leiomyoma
Malignant
Leiomyosarcoma (excluding skin)
There are—however—some intramuscularly located tumors (e.g., usual lipoma) that may be characterized with confidence on MR imaging. Lesions with more or less specific imaging features are mostly benign, whereas malignant soft tissue tumors (STT) do not have specific imaging features. Although histologically not belonging to the group of muscle tumors (according to the World Health Organization Classification 2013; Table 1), the imaging features of characteristic STT located within the muscle will be briefly reviewed.
Furthermore, this chapter will deal with the most frequent muscular pseudotumors.
Finally, tumor-like conditions of fasciae and tendon sheaths will be discussed.
Muscle tumors involving the gastrointestinal system, the retroperitoneum, uterus, and other organ systems different from the musculoskeletal system are excluded.
3 Benign Muscle Tumors
3.1 Leiomyoma
3.1.1 Definition, Clinical Findings, and Histology
Superficial leiomyoma
Cutaneous leiomyomata (leiomyoma cutis) arise from the arrector pili muscle of the skin. They present as clustered papules or less commonly as single painful nodules. The size ranges from a few millimeters up to 2 cm.
Lesions arising from the deep dermis of the scrotum, nipple, areola, and vulva are designated as genital leiomyoma (Kransdorf and Murphey 2007).
Angioleiomyoma is a rare benign tumor, arising from the muscularis media of small vessels (Hachisuga et al. 1984). It is currently classified as a pericytic tumor rather than a true smooth muscle tumor (Fletcher et al. 2013). There are three histopathological subtypes: solid, cavernous, and venous.
The lesion is most common in adults, with two-thirds occurring in the fourth through sixth decades of life. The tumor is typically small (less than 2 cm) and often slowly growing. It may be localized in the dermis, the subcutaneous fat, or the superficial fasciae of the extremities (Vanhoenacker et al. 2009) (Fig. 1).
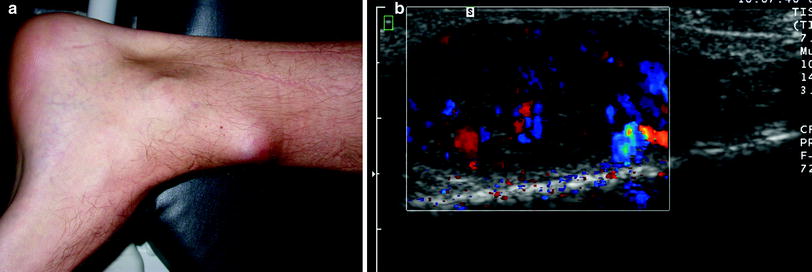

Fig. 1
Angioleiomyoma. a Photograph of a 34-year-old man presenting with a slowly growing mobile soft tissue mass at the left lower leg. b Longitudinal ultrasound showing a well-defined hypoechoic lesion within the subcutaneous tissue. The lesion is vascular on power Doppler
It is painful in over half of the cases (Ramesh et al. 2004). Classically, tenderness and pain, which is often paroxysmal in nature and precipitated by light touch or changes in temperature, are the presenting features in 50–70 % of cases (Gupte et al. 2008).
Deep leiomyoma
Leiomyomata of the deep soft tissue are very rare in comparison with superficial leiomyomata. Furthermore, long-term follow-up showed that deep leiomyoma may demonstrate aggressive behavior, leading to the assumption that these lesions could be potentially malignant (Weiss 2002). Therefore, histological confirmation of the lesion is mandatory to make a reliable diagnosis (Billings et al. 2001).
There are two distinct subtypes: the deep leiomyoma of extremities (the somatic leiomyoma) and the retroperitoneal or abdominal leiomyoma (Kransdorf and Murphey 2007).
3.1.2 Imaging Findings
Superficial leiomyoma
Cutaneous leiomyomata are dermatological-based lesions which are rarely referred for imaging (Davies et al. 2010).
Conventional radiographs of angioleiomyoma are mostly inconclusive. Rarely, they may show scalloping of the cortex of the adjacent bone or intralesional dystrophic calcifications (Vanhoenacker et al. 2009).
On ultrasound, the tumor is homogeneously hypoechoic, with well-defined margins and without contact with the superficial fascia. Typically, the tumor is oval shaped with the longest axis parallel to the extremity axis. Color Doppler reveals hypervascularity (Vanhoenacker et al. 2009) (Fig. 1b).
The three histopathological subtypes (solid, cavernous, and venous subtypes) all display similar MR appearance (Fig. 2).


Fig. 2
Angioleiomyoma. a Axial T1-Weighted Image (WI). Well-delineated subcutaneous lesion abutting the medial aspect of the Achilles tendon. The lesion is isointense compared to muscle. b Coronal fatsuppressed (FS) T2-WI. Note lesion heterogeneity with areas of high and low signal intensity (white arrow). c Axial FS T1-WI after intravenous (IV) administration of gadolinium contrast. There is marked but slightly inhomogeneous enhancement
On T1-weighted images (WI), the lesion appears either homogeneously or heterogeneously isointense to skeletal muscle. On T2-WI, it is mainly hyperintense with few low signal intensity (SI) foci. The smooth muscle cells and patent vessels are believed to correspond to the hyperintense areas, whereas the low SI foci correlate with fibrous tissue or intravascular thrombi within the mass (Gupte et al. 2008). The lesion shows vivid enhancement after administration of intravenous gadolinium contrast. Hyperintense areas on T2-WI show strong enhancement, whereas the low SI foci on T2-WI enhance less (Ramesh et al. 2004) (Fig. 2).
Deep leiomyoma
Radiographs or CT may occasionally show intralesional calcifications of variable morphology (either sandlike, plaquelike, or large mulberry pattern) (Davies et al. 2010; Kransdorf and Murphey 2007).
3.2 Rhabdomyoma
3.2.1 Definition and Clinical Findings
Rhabdomyoma is a rare benign tumor composed of striated muscle cells (Davies et al. 2010). There are two broad categories, cardiac and extracardiac rhabdomyoma. Cardiac rhabdomyoma is associated with the tuberous sclerosis complex. Extracardiac rhabdomyoma is further subdivided into adult, fetal, and genital subtypes.
The adult subtype is a slowly growing painless mass most typically seen in the neck and rarely in the somatic skeletal muscle (Kransdorf and Murphey 2007).
3.2.2 Imaging
Cardiac rhabdomyoma are usually detected on echocardiography as solitary or multiple hyperechoic mass(es) within the myocardium, whereas reports of ultrasound (US) features of extracardiac rhabdomyoma are scarce. Maglio et al. (2012) reported a case of nonspecific oval hypoechoic mass in the neck of an adult patient. MR imaging reveals a well-delineated mass isointense or hyperintense compared to skeletal muscle on T1- and T2-WI. Central necrosis and hemorrhage is rarely seen. After administration of intravenous gadolinium contrast, there is marked homogeneous or heterogeneous enhancement (Davies et al. 2010; Kransdorf and Murphey 2007).
3.3 Other Benign STT with Intramuscular Location
3.3.1 Intramuscular Lipoma
3.3.1.1 Definition
Lipomas are well-capsulated masses composed of mature adipose cells.
Although lipomas are most frequently superficially located, they may also be located intramuscularly.
3.3.1.2 Imaging
Radiographs are usually normal, but a low to intermediate density may be seen in larger lesions.
On ultrasound, intramuscular lesions are often more difficult to distinguish from the surrounding muscle fibers. The reflectivity is highly variable ranging from hypo- to hyperreflective compared to subcutaneous fat (Fig. 3a).
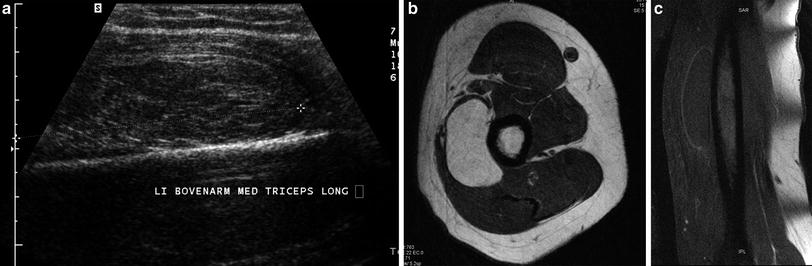

Fig. 3
Intramuscular lipoma in the left triceps muscle. a Longitudinal ultrasound showing a slightly hyperechoic lesion within the medial head of the triceps muscle. The lesion is difficult to distinguish from the surrounding muscle fibers. b Axial T1-WI. In contradistinction to the ultrasound findings, the lesion is well demarcated from the surrounding muscle and is isointense to subcutaneous fat. c Coronal FS T1-WI after IV administration of gadolinium contrast. There is fat suppression of the lesion and there is only faint peripheral enhancement of the compressed capsule
CT shows a homogeneous mass with low density (−60 to −130 HU). Intramuscular lesions may contain thin fibromuscular septa. There is no significant contrast uptake.
On MRI, the lesion has similar signal characteristics as subcutaneous fat on all pulse sequences. Fat-suppression techniques show homogeneous suppression. Minor (less than 2 mm) internal septa and a low SI capsule may be seen. After administration of intravenous gadolinium contrast, there is no contrast enhancement, except for the peripheral fibrous capsule or the subtle internal septa (Vanhoenacker et al. 2006) (Figs. 3 and 4).
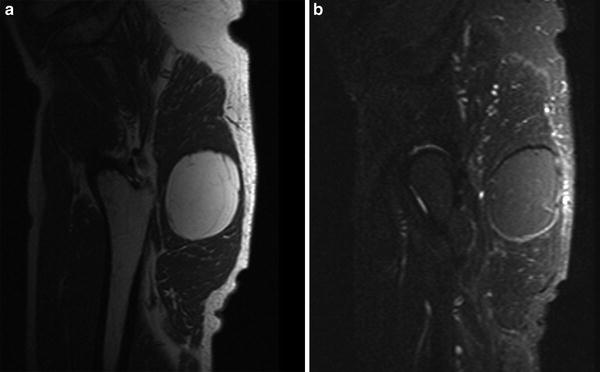

Fig. 4
Intramuscular lipoma in the gluteus maximus muscle. a Sagittal T1-WI. The lesion is isointense to subcutaneous fat except for some subtle intralesional strands of low signal, corresponding to small residual muscle fibers. b Sagittal FS T1-WI after IV administration of gadolinium contrast. There is fat suppression of the lesion and there is no significant contrast enhancement
Table 2 summarizes the most useful criteria to differentiate between a usual lipoma and a liposarcoma.
Table 2
Differential diagnostic criteria on MRI between lipoma/liposarcoma
Lipoma | Liposarcoma | |
|---|---|---|
Location | Subcutaneous fat or muscle | Deep location (intramuscular, retroperitoneum) |
Size | Less than 5 cm | Larger than 5 cm |
Shape | Round, oval or fusiform | Multilobulated |
Contents | Homogeneous fat-like or thin internal (fibromuscular) septa less than 2 mm | Inhomogeneous with intralesional non fat containing noduli or septa thicker than 2 mm |
Enhancement | No enhancement | Intralesional foci of enhancement |
3.3.2 Intramuscular Myxoma
3.3.2.1 Definition
Myxoma is histologically characterized by the presence of abundant, avascular, and myxoid stroma in which a small number of cells are embedded. Most myxomas are intramuscularly located (82 %). It is a tumor of adults. Areas most frequently involved are the large muscles of the thigh (Fig. 5), shoulder, buttocks, and upper arm. There is an association with polyostotic fibrous dysplasia (bones involved are usually in the vicinity of the myxoma) in Mazabraud’s syndrome (De Schepper and Bloem 2007) (Fig. 6).
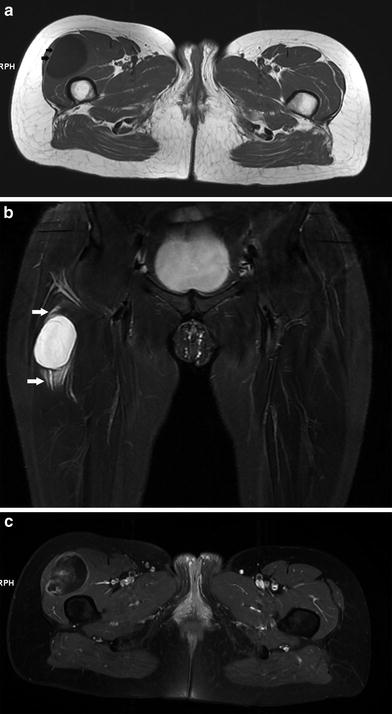
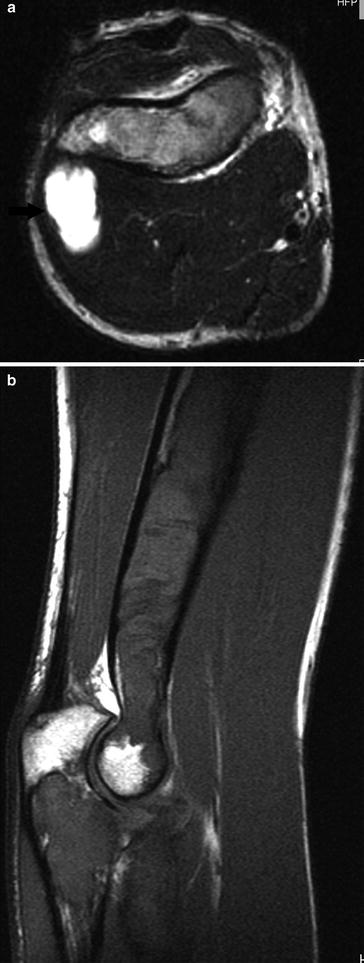

Fig. 5
Intramuscular myxoma in the right quadriceps muscle. a Axial T1-WI. The lesion is hypointense compared to muscle. Note a small peripheral rim of fat at the lateral aspect of the lesion, demarcating the lesion from the tensor fasciae latae muscle (black arrows). b Coronal FS T2-WI. The lesion is of high signal. Perilesional strands of high signal are caused by leakage of myxomatous tissue in surrounding muscle (white arrows). c Axial FS T1-WI after IV administration of gadolinium contrast. Note a “smoke”-like enhancement pattern

Fig. 6
Intramuscular myxoma associated with Mazabraud syndrome. a Axial T2-WI. Well-delineated intramuscular lesion within the lateral head of the right biceps muscle. The lesion is hyperintense compared to fat. b Sagittal T1-WI of the right elbow. Note bone expansion and abnormal bone marrow SI isointense to skeletal muscle within the distal humerus and proximal diaphysis of the ulna, in keeping with fibrous dysplasia
3.3.2.2 Imaging
Ultrasound shows a well-delineated solid or mixed hypoechoic lesion with fluid-filled clefts or internal cystic foci reflecting myxoid stroma. Usually, there is retroacoustic sound enhancement. A small amount of hyperechoic fat or edema may surround the upper and lower poles of the lesion (Zamorani and Valle 2007). On MRI, myxomas present as very low signal intensity lesions on T1-weighted images (lower than signal intensity of normal muscle) and as high signal intensity lesions on T2-WI (higher than signal intensity of fat). A small rim of fat corresponding to focal atrophy of surrounding muscle may be seen on T1-WI (Luna et al. 2005). On T2-WI, perilesional strands of high signal may be seen due to leakage of myxomatous tissue in surrounding muscle (Nishimoto et al. 2004). There may be moderate, central enhancement after administration of intravenous gadolinium contrast, having a “smoke”-like appearance (Fig. 5) (Peterson et al. 1991).
3.3.3 Desmoid Tumors (desmoid type fibromatosis)
3.3.3.1 Definition and Clinical Findings
Extra-abdominal desmoids are rare benign soft tissue tumors arising from connective tissue of muscle, overlying fascia, or aponeurosis. They have been designated previously as desmoid tumor, aggressive fibromatosis, or musculoaponeurotic fibromatosis. The term “desmoid” means band-like or tendon-like lesions (De Schepper and Vandevenne 2006). Currently, the term desmoid type fibromatosis is used (Fletcher et al. 2013). The lesions can be regarded as deeply seated fibromatosis in contradistinction to palmar fibromatosis which is superficially located. Desmoids are infiltrative tumors, known for their frequent recurrences. Complete surgical excision is often difficult.
The most commonly encountered locations are upper arm (28 %), chest wall and paraspinal area (17 %), thigh (12 %), neck (8 %), knee (7 %), pelvis or buttock (6 %), and lower leg (5 %). Desmoids occur most frequently in patients in the second to fourth decades of life, with a peak incidence between the ages of 25–35 years (Murphey et al. 2009). The lesion is typically deeply seated and poorly circumscribed. Slow insidious growth is the rule and most lesions are painless (Murphey et al. 2009).
3.3.3.2 Imaging
Plain radiographs are often normal. A nonspecific soft-tissue mass may be apparent in patients with larger lesions. Calcification is uncommon. Underlying pressure erosion or cortical scalloping may be seen.
Ultrasound may demonstrate a nonspecific poorly defined, hypoechoic soft-tissue mass. Large lesions may demonstrate posterior acoustic shadowing. Increased vascularity is seen on Doppler-ultrasound (Murphey et al. 2009; Chew and Vanhoenacker et al. in press) (Fig. 7a).
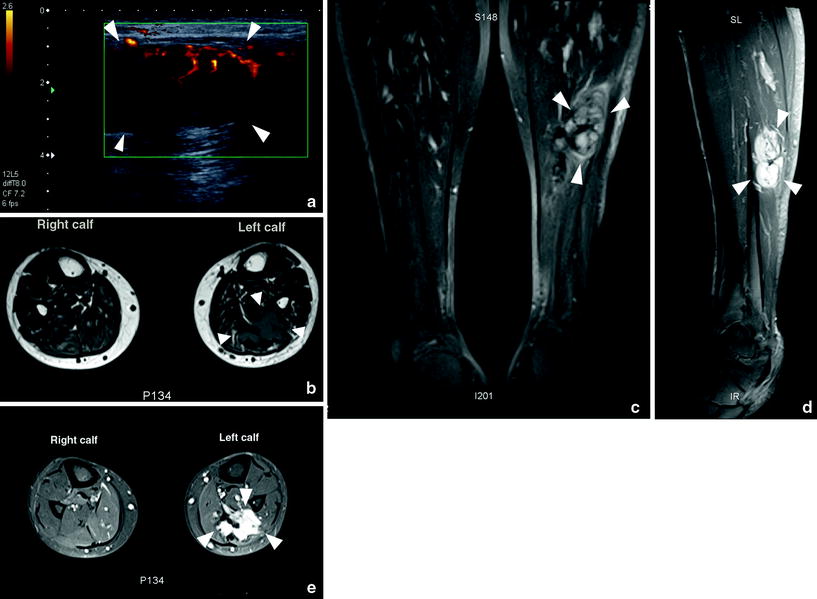

Fig. 7
Intramuscular desmoid of the calf muscles. a Ultrasound with power Doppler. A solid hypoechoic lesion (arrowheads) with peripheral neovascularity and posterior acoustic shadowing is identified. b Axial T1-WI. Infiltrative lesion (arrowheads) within the left lateral gastrocnemius lesion. The lesion invades the soleus muscle anteriorly and the medial gastrocnemius medially. The lesion is of intermediate signal. c Coronal FS T2- WI. The lesion (arrowheads) is hyperintense. Note its central hypointensity and irregular superior margins. Central hypointensity likely correlated to dense fibrous tissue. d Sagittal FS T1-WI after IV administration of gadolinium contrast. There is avid enhancement of the lesion (arrowheads). Note the irregular infiltrative superior margins. e Axial FS T1-WI after IV administration of gadolinium contrast. There is avid enhancement of the infiltrative lesion (arrowheads). (Used with permission from Chew NS et al. JBR-BTR in press)
On CT, the lesion has variable attenuation and enhancement (up to 110 HU). The attenuation is usually similar to that of skeletal muscle, but lesions with more prominent collagen content may reveal mildly higher attenuation. Low attenuation is rare and is associated with myxoid components. The margins are often indistinct (Murphey et al. 2009).
On MRI, the lesion may be well demarcated or ill defined. The signal intensity of desmoid type fibromatosis is variable, and depends on the extent of collagen and degree of cellularity of the lesion. Low signal intensity areas on T2-WI correspond histologically to hypocellular and collagen-rich portions of the tumor, whereas more cellular areas with a large extracellular space are of high signal on T2-WI. In addition, high T2 signal may be seen in myxoid portions.
3.3.4 Intramuscular Haemangioma and Vascular Malformations
Vascular anomalies of the soft tissues comprise a heterogeneous spectrum of lesions, encompassing hemangiomas, and vascular malformations. Although the term “hemangioma” should be strictly reserved for a group of benign endothelial neoplasms consisting of cellular proliferation and hyperplasia occurring in children (Flors et al. 2011), the terms hemangioma and vascular malformation are often used interchangeably in daily practice.
Hemangiomas may involve superficial (cutis and subcutis) and deep compartments (such as intramuscular location).
Plain radiographs and CT may show intralesional phleboliths.
US shows a complex ill-defined mass within the affected muscle, characterized by a mixtures of hypo-or anechoic (corresponding to blood-filled cavities) and hyperechoic (reflecting fat) components. Intralesional phleboliths may appear as bright dots with retroacoustic shadowing. Doppler imaging may distinguish high-flow and slow-flow vascular malformations (Zamorani and Valle 2007).
On MRI, hemangiomas are characterized by their high signal intensity on T2-WI and intermediate (between that of muscle and fat) signal intensity on T1-WI. They are frequently multilobular, resembling a “bunch of grapes”. Fluid–fluid levels may be seen in cavernous hemangiomas. A peripheral fat induction phenomenon is a frequent feature of intramuscular hemangiomas. High-flow lesions may show signal voids on all pulse sequences. After administration of intravenous gadolinium contrast they exhibit a serpiginous, marked enhancement. In low-flow venous hemangiomas, large venous convolutes are associated with late enhancement (De Schepper and Bloem 2007) (Fig. 8).
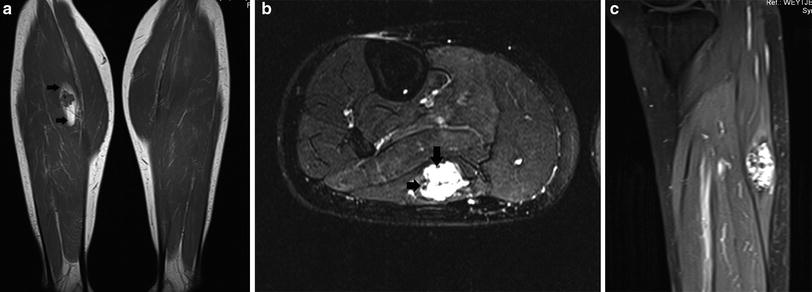

Fig. 8
Intramuscular hemangioma in the right lateral gastrocnemius muscle. a Coronal T1-WI. The lesion is predominantly isointense compared to muscle with some intralesional low SI foci (phleboliths). Note a peripheral rim of high signal intensity, due to fat induction (black arrows). b Axial FS T2-WI. The lesion is predominantly hyperintense with some low-signal intensity foci, in keeping with phleboliths (black arrows). c Sagittal FS T1-WI after IV administration of gadolinium contrast. There is marked enhancement of the lesion, except for the intralesional phleboliths. Note the lobulated appearance of the contour of the lesion, resembling a bunch of grapes
4 Malignant Muscle Tumors
Malignant tumors with muscle differentiation are summarized in Table 1.
Extrauterine leiomyosarcomata account for 9 % of all soft tissue tumors and are typically seen in adults (Davies et al. 2010).
Rhabdomyosarcoma is the most common soft tissue tumor in children (Navarro 2011).
Many malignant soft tissue tumors that are identified on imaging to arise in muscle do not show muscle differentiation histologically. On MRI, these lesions are often indistinguishable from STT with myogenic differentiation.
Table 3 summarizes the important MR parameters which may suggest malignancy, irrespective of the histological composition of the lesion.
Table 3
General MR imaging features that may suggest malignancy (modified from De Schepper and Bloem 2007)
Large volume (any lesion exceeding 3 cm) |
Ill-defined margins |
Inhomogeneity on all pulse sequences |
Intralesional hemorrhage |
Intralesional necrosis |
Extensive and peripheral enhancement pattern (with papillary projections) on static contrast examination |
Rapid enhancement with steep slope on dynamic contrast examination |
Extracompartmental extension |
Invasion of adjacent bones and neurovascular bundles |
4.1 Leiomyosarcoma
4.1.1 Definition and Clinical Findings
Extrauterine leiomyosarcomata typically occur in adults. There are four main subtypes, including cutaneous, subcutaneous, deep soft tissue, and vascular leiomyosarcoma. Cutaneous leiomyosarcoma has a far better prognosis than the subcutaneous type.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree