(1)
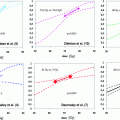
D1, D2, and D3 are isoeffective total doses, e.g., doses which cause 50 % of local tumor control (TCD-50); d1, d2, and d3 are the respective doses per fraction; α/ß is a tissue, effect, or tumor-specific constant.
The validity of the α/ß value derived this way depends crucially on the condition that except for the different dose fractionations under study all other conditions in both arms of the fractionation study are equal, in particular tumor size and stage, follow-up and treatment outcome criterion, dose specification, other treatments and, above all, overall treatment time. This is difficult to achieve in human tumors if retrospective studies are analyzed but can be precisely achieved in experiments using transplanted mouse tumors.
Using this method, α/ß ratios have been determined for a large number of experimental tumors, in particular isogenic mouse tumors. These values have been collated by Williams et al. (1985). Values of α/ß ratios from 48 different experimental tumors ranged from 1 to 35 Gy with a mean value of approximately 10 Gy. Based on this review, it is generally recommended that in case no specific data are available for the particular tumor under consideration, a default α/ß value of 10 Gy should be used for tumors.
3 The Dependence of Human Tumor Cure Doses on the Dose Per Fraction: The Alpha/Beta Ratio of Human Cancers, in Particular of Prostate Cancer
The number of clinical studies which permit the reliable analysis of α/ß values is limited. In skin cancers, Trott and colleagues calculated a value of about 10 Gy (Trott et al. 1981). Several studies in squamous cell carcinomas of the head and neck found similar results (Stuschke and Thames 1999). However, there was considerable variation due to the fact that the different tumor groups differed with regard to size, dose distribution, or overall treatment time.
The first cancer in which clinical radio resistance was attributed to a high fractionation sensitivity/low α/ß value was malignant melanoma. In vitro, large variation between different cell lines isolated from human malignant melanomas with α/ß values ranging from 1 to 50 Gy and SF2 values ranging from 0.15 to 0.75 have been observed (Rofstad 1986). Studies of transplantable mouse melanomas yielded α/ß values between 6 and 14 Gy. Early retrospective evaluation of clinical fractionation schedules yielded conflicting results by either showing a higher (Habermalz 1981) or lower (Trott et al. 1981) rate of complete response rate with higher doses per fraction. In the largest retrospective study on fractionation sensitivity an α/ß ratio of 2.5 Gy was estimated (Overgaard et al. 1986), which was later recalculated to be equal to 0.6 Gy (Bentzen et al. 1989). Finally, a randomized clinical study performed on 126 patients with metastatic melanomas comparing the results of four fractions of 8 Gy with 20 fractions of 2.5 Gy did not record any difference in outcome (Sause et al. 1991). In conclusion, the retrospective analyses of response rates of melanomas to different fractionation protocols have produced conflicting results rendering reliable α/ß estimation impossible.
More recently, a low α/ß ratio for breast cancer has been postulated by Whelan et al. (2002). This lead to a large randomized clinical study called START, which is still in progress but various interim reports have been published (Haviland et al. 2013).
To date, the most important retrospective clinical analyses on fractionation sensitivity of human cancers are those on prostate cancer and a wealth of α/ß estimates have been published since then.
In vitro experiments and calculation of PSA doubling times after external beam radiotherapy from clinical data yielded a slow cell cycle time for prostate cancer compared to other tumor types (Pollack et al. 1994; Haustermans et al. 1997). Based on the assumption that the potential cell doubling time of a particular tumor is associated with the fractionation sensitivity, the experimental findings were considered not compatible with a presumed α/ß ratio >8 Gy. This notion formed the initial basis to reconsider the presumed α/ß ratio of prostate cancer and resulted in the seminal work by Brenner and Hall (1999) as well as Fowler et al. (2001) to estimate and derive a α/ß ratio from retrospective clinical data. As detailed above, in order to calculate a α/ß value, isoeffective fractionation schedules have to be employed. Brenner and Hall used two datasets on isoeffective treatments from external beam radiotherapy and low dose rate iodine seed brachytherapy to arrive at an estimated α/ß value of 1.5 Gy (95 % CI 0.8–2.2 Gy). Extending this approach to include isoeffective LDR implant strategies with 103-Paladium (Fowler et al. 2001) and to then available hypo-fractionated data (Chappell et al. 2004), a similar α/ß ratio around 1.5 Gy was established again.
Still, one has to bear in mind that in order to derive a α/ß value with this approach simplified assumption were made the most relevant will be discussed briefly.
To eliminate the ß component in their calculation and for the lack of available hypo-fractionation datasets, Brenner and Hall (1999) used data from LDR brachytherapy where complete sublethal damage repair could be assumed. Though elegant in principle, a completely different dose distribution with inherent dose heterogeneity and dose rate in LDR brachytherapy was compared to homogenous EBRT, all factors differently affecting biologically effective dose. It was even reported that implantation edema may negatively affect dose rate and hence BED (Van Gellekom et al. 2002).
Looking at the derived parameters for α and ß, the assumed number of clonogenic cells was claimed to be considerably underestimated and not congruent between LDR and EBRT.
However, each of these retrospective analyses were based on the assumption that repopulation during a course of radiotherapy of prostate cancer can be neglected and that there was no “time factor.” As repopulation in prostate cancer with an assumed T pot of 42 days was not considered relevant during a course of normo-fractionated radiotherapy, it had not been taken into account. Still, at least with protracted treatments such as LDR brachytherapy it should have been considered.
Though criticized for uncertainties to the assumption inherent to this approach, these initial analyses stirred a wealth of research interest, especially as data of hypo-fractionated regimens became available and were incorporated to overcome the limitations in comparing EBRT and BT datasets.
To overcome some of the limitations when using brachytherapy datasets, several groups compared large EBRT only patients cohorts and calculated α/ß values between 1.4 and 3.7 (Williams et al. 2007; Proust-Lima et al. 2011; Leborgne et al. 2012).
Interestingly, no difference of α/ß ratios between the different prostate cancer risk groups were found so far (Nickers et al. 2010; Miralbell et al. 2012).
The analysis of a nine-institution database of nearly 5,000 patients treated for prostate cancer with different radiotherapy schedules by Thames et al. (2010) proved that overall treatment times is a significant determinant of outcome of radiotherapy in low and intermediate-risk patients treated to 70 Gy or higher. A time factor equivalent to 0.24 Gy/d was estimated. This lead Baumann et al. (2010) to suggest that the apparent low α/ß ratio was artificially caused by the different overall treatment times of hypo-fractionated and the conventional treatment schedules. Vogelius and Bentzen (2012) estimated a mean time factor from several studies of 0.3 Gy/day. Incorporating this time factor into the analysis of the α/ß value also correcting for the lack of isoeffectiveness of the different schedules using a logistic dose response with a gamma-50 of one increased the α/ß value from close to zero to 4 Gy (Table 1).
Table 1
Published α/ß estimates based on clinical data comparing different fractionation schedules either as external beam radiotherapy (EBTRT) or high-/low-dose brachytherapy (HDR-BT or LDR-BT)
Author | Radiation treatment | Estimated α/ß value (95 % CI) |
|---|---|---|
Brenner and Hall (1999) | EBRT, LDR-BT | 1.5 Gy (0.8–2.2 Gy) |
King et al. (2000) | EBRT, LDR-BT | 4.96 Gy (4.1–5.6 Gy) |
Fowler et al. (2001) | EBRT, LDR-BT | 1.49 Gy (1.25–1.76 Gy) |
Brenner et al. (2002) | EBRT + HDR-BT | 1.2 Gy (0.03–4.1 Gy) |
Wang et al. (2003)a | EBRT + HDR-BT | 3.41 Gy (2.56–4.26 Gy) |
Chappell et al. (2004) | EBRT, LDR-BT | 1.44 Gy (1.22–1.76 Gy) |
Bentzen et al. (2005) | EBRT | 1.12 Gy (3.3–5.6 Gy) |
EBRT (Hyperfractionation) | 8.3 Gy (0.7–16) | |
Wiliams et al. (2007) | all pts. | 2.6 Gy (0.9–4.8 Gy) |
EBRT pts. only | 3.6 Gy (0.9–∞) | |
Nickers et al. (2010) | EBRT | 3.41 Gy (2.56–4.26 Gy) |
Shaffer et al. (2011) | EBRT, LDR | >30 Gy (5.5–∞) |
Proust-Lima et al. (2011) | EBRT only | 1.55 Gy (0.46–4.52 Gy) |
Nickers et al. (2010) | EBRT | 3.41 Gy (2.56–4.26 Gy) |
Leborgne et al. (2012) | EBRT | 1.86 Gy (0.7–5.1) |
Miralbel et al. (2012) | EBRT | 1.4 (0.9–2.2 Gy) |
Vogelius and Bentzen (2012)a | EBRT | 1.93 Gy (0.27–4.14) |
Recalculations taking the different confounding factors into consideration still mostly arrived at α/ß ratios below 5 Gy.
In conclusion, with regard to the available data the α/ß value of prostate cancer indeed seems considerably lower compared to other common tumor entities and may be assumed to be at around 3–4 Gy. Therefore, we regard the α/ß value derived by Vogelius and Bentzen (2012) as the most reliable figure, which we will use in the discussion of the radiobiological justification of hypo-fractionated radiotherapy of prostate cancer.
Due to the inherent uncertainties in the derivation of the α/ß ratio in all theoretical analyses of retrospective data, a reliable α/ß estimate with narrower confidence intervals is only to be expected from large prospective randomized trials comparing different EBRT fractionation schedules with sufficient long-term follow-up but this is unlikely to be available in the near future.
4 The Fractionation Sensitivity of Critical Normal Tissues in Radiotherapy
The radiobiological justification of hypo-fractionated radiotherapy rests on the assumption that the fractionation sensitivity or α/ß ratio of the cancer tissue is significantly lower than that of the critical complications in the normal tissues inevitable exposed to high radiation doses. In their seminal work, Brenner and Hall (1999) estimated an α/ß value of prostate cancer tissue of 1.5 Gy and an α/ß value for rectal complications of 5 Gy. If these values would be correct, there would not be any further discussion on the radiobiological basis of hypo-fractionation in radiotherapy of prostate cancer. But there are serious doubts on the validity, not only of the fractionation sensitivity of the cancer tissue but even more so on the validity of the value for rectal complications.
One of the most critical normal tissues to be considered in the optimization of prostate cancer radiotherapy is the recto-sigmoid, the rectum, and anal canal. In clinical practice, severity scores are used to report treatment outcome but these scores are a mixture of different manifestations of late radiation damage. These scores have been developed, e.g., by the RTOG to compare severity and rates of complications from different treatment protocols, however, they are of very limited value for clinical radiobiology research such as estimating α/ß ratios (Trott et al. 2012). Four different types of symptoms following the development of late normal tissue damage in the rectum of patients after treatment for prostate cancers have been identified, namely rectal bleeding, urgency/frequency, tenesmus, and fecal incontinence (Trott et al. 2012). Each of these symptoms is likely to be caused by a different pathogenic mechanism; each of them is also related to a different distribution of the radiation dose in the rectum. This means that volume-based or surface-based NTCP models which have been used to normalize dose distributions are of very limited value for clinical research. The severity of bleeding is related to the area of the rectal wall which exceeds a threshold dose. Urgency and frequency are also related to the area of rectal mucosa which develops severe early reactions. However, a different geometric dose distribution is likely to influence severity. Fecal incontinence is an effect, which depends on the radiation dose in the anal sphincter. Experimental data suggest that not only does the critical target area/volume differ between the different complication types, but also the effects of dose per fraction are likely to be different (Trott et al. 1981). Whereas for telangiectasia, the α/ß ratio is low and may be <3 Gy, it is higher for the chronic inflammation causing fibrosis. No data are available for sphincter damage. To use a single value for the four different radiobiological mechanisms of potential ano-rectal complications of radiotherapy for prostate cancer as it has been done in the justification of hypo-fractionation in radiotherapy for prostate cancer is certainly inadequate.
Several studies used results from randomized and nonrandomized clinical studies comparing hypo-fractionation and conventional fractionation in radiotherapy of prostate cancer to estimate α/ß values (Marzi et al. 2009; Tucker et al. 2011; Leborgne et al. 2012) and all arrived at values only little smaller than the original estimate of Brenner and Hall (1999). This is probably due to the fact that all used essentially the same approach, comparing incidence of RTOG scores of grade 2 or more. The confidence limits of these estimates were high, including the accepted value of the α/ß ratio for the prostate cancer tissue. The main aim is to prevent quality of life impairing late complications (e.g., greater or equal than grade 3). However, complications of this severity are rare (<1 % of all patients, whereas the rate of grade 2 complications is >10 %). It is for this reason that the moderate severity complications are used for analysis hoping that the fractionation sensitivity of severe complications is the same as that for moderate severity complications but there is no scientific evidence supporting this assumption. It is obvious that a direct estimate of the fractionation sensitivity of severe rectal complications cannot be derived from clinical trials data alone, for the various reasons listed above.
There are only few experimental data available which could be used to assist in this task. Dubray and Thames (1994) analyzed the existing data from animal experiments on the response of the rodent rectum to fractionated or low dose rate exposure, in particular the experiments performed by the Munich group and summarized by (Trott et al. 1981). They concluded that the α/ß value was about 4–5 Gy, similar to the values used in the justification of hypo-fractionated treatment schedules. However, Kummermehr and Trott (1994) pointed out that these values are the result of pooling data, which are related to very different radiobiological mechanisms. A large proportion of “late” normal tissue damage in patients as well as in rats, such as ulceration, severe telangiectasia, severe fibrosis is a typical consequential late normal tissue damage which is associated with a very different fractionation sensitivity, i.e., much less than “genuine” late normal tissue damage. Pooling fractionation data from both clinical endpoints into one endpoint is likely to yield an alpha beta value half-way between that for early damage and late damage. Kummermehr and Trott (1994) warned that pooling late normal tissue effects which may not represent the response of the same tissue component in the various treatment protocols may produce unsafe results. A major factor in this interaction of early and late normal tissue damage is related to regenerative processes such as repopulation which crucially depends on overall treatment time. Also Dubray and Thames (1994) warned to combine early and late injury of the rectum in order to produce the clinical endpoint (which may be telangiectasia, fibrosis, chronic inflammation) and that therefore an increase of “late” rectal radiation damage might be expected to occur with decrease in overall treatment time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree