Fig. 13.1
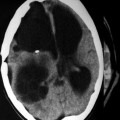
In vivo 1H MRS of the normal brain. Predominantly, three metabolites are seen at TE = 135 ms. These are NAA, Cho and Cr, NAA being the largest, seen at 2 ppm
Lactate (1.3 ppm) is a nonspecific marker often associated with anaerobic conditions in different situations. The configuration of lactate resonance consists of two distinct, resonant peaks (called a ‘doublet’) that are due to magnetic field interactions between adjacent protons (J-coupling) (Fig. 13.2). Lactate levels in the brain are normally very low or absent (Fig. 13.1). The presence of lactate indicates that the normal cellular oxidative respiratory mechanism is no longer in effect and that carbohydrate catabolism is taking place. At time to echo, TE = 272 ms, the lactate doublet projects above the baseline, whereas at TE = 136 ms, the lactate doublet is projected below the baseline (Castillo et al. 1998).


Fig. 13.2
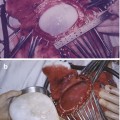
In vivo 1H MRS showing typical lactate peak in a necrotic mass (a) shows MRS with TE = 135 ms and (b) shows MRS with TE = 270 ms. The typical lactate peak is a doublet, projecting below baseline in (a) and above baseline in (b)
The results of MRS are compared and evaluated in various ways. The signal intensities of the metabolites are taken as a ratio against a standard metabolite, which is least likely to change (e.g. Cr in case of 1H MRS). These ratios can be compared by taking the other side as control. Several quantitative methods are also used. The observed signal intensity is multiplied by the 90° pulse voltage, thereby compensating for differences in radiofrequency coil loading, and then comparing the signal intensities between different subjects. Using MRS, the concentration of metabolites can also be measured by determining the effects of T1 and T2 relaxation. Another method is that the signal intensity can be calibrated against a standard (phantom) (Gadian et al. 1994; Duncan 1997; Hunter and Wang 2001).
Cysts and Cyst-like Lesions of the Brain
A variety of cysts and cystic lesions can be seen, including arachnoid cysts, porencephalic cysts, neuroglial cysts, epidermoids, large perivascular spaces, cyst or necrosis associated with tumours, abscesses, parasitic cysts such as hydatid and neurocysticercosis (NCC), etc. (Osborn and Preece 2006). While history, clinical features, geographical factors and conventional CT and MRI appearances are often helpful in differentiating these lesions, the appearances are occasionally atypical and can present with diagnostic dilemmas. Since these represent vastly different pathologies requiring different treatment approaches, the importance of accurate diagnosis cannot be overemphasized. 1H MRS utilizes a different, metabolite-based approach and provides a vital clue in several of these difficult situations.
Proton Magnetic Resonance Spectroscopy in Hydatid Cysts
Biochemical Basis of Magnetic Resonance Spectroscopy
In parasitic cysts, some anaerobic metabolism is usually present, even if the aerobic pathway is active to some extent (Garg et al. 2002). As a result, a variety of metabolites involved in anaerobic metabolism are usually found. Of the parasitic cysts, hydatid cyst is a typical example that shares many similarities and some differences with other parasitic cysts. The frequently described metabolites found in hydatid fluid in reasonable amounts are succinate, pyruvate, acetate, lactate and alanine, while a few others such as malate, fumarate, glycine, other amino acids, Cho (and Cho-containing compounds), propionate, etc. are found in smaller amounts or traces (McManus and Smyth 1978; Chang et al. 1998; Garg et al. 2002; Jayakumar et al. 2003). The frequency and success of detection of metabolites depend largely on the technique used.
Pyruvate is a product of glycolysis and reported to be found in abundance in hydatid fluid; investigators have confirmed its presence using mass spectroscopic measurements (Jayakumar et al. 2003). It is also thought to be an indicator of viability as it has not been detectable in cysts that show degeneration on histopathologic examination (Jayakumar et al. 2003). Pyruvate can be metabolized to acetate and lactate which are important and easily detectable metabolites in hydatid fluid, lactate being more prominently seen. Under anaerobic conditions, the partial tricarboxylic acid (TCA) cycle is active, in which phosphoenolpyruvate formed during glycolysis partially converts into oxaloacetate and then further into malate, using malate dehydrogenase (MDH). It further transforms into fumarate and succinate (McManus and Bryant 1995; Garg et al. 2002). In hydatid, the activity of MDH and other related enzymes has been reported as more favourable for succinate production by some of the investigators (Garg et al. 2002), who regard succinate as a very important metabolite present in significant amount in hydatid cysts and have confirmed its presence by specialized techniques (Garg et al. 2002). However, other investigators have given more importance to pyruvate, describing it as a marker of cestodial infestation and have downplayed the importance of succinate by citing low biochemical activity of succinate dehydrogenase (Jayakumar et al. 2003). Therefore, in the literature, there is no consensus on the relative importance of succinate and pyruvate, and as discussed below, it has important ramifications for 1H MRS.
The only significant amino acid found is alanine, which is derived from pyruvate (Kohli et al. 1995; Jayakumar et al. 2003), while others such as leucine, valine and isoleucine are found in small amounts. Fumarate and malate are only detectable in traces but are considered as important metabolites that indicate fertility and can also be useful for differentiation from NCC cysts (Garg et al. 2002).
Technical Factors of In Vivo Proton Magnetic Resonance Spectroscopy
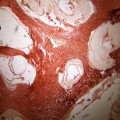
While both PRESS and STEAM techniques have been used, PRESS is more commonly performed. Both SVS and CSI techniques have been successfully used. At 1.5 T, different researchers use (time to repeat) TR of 1,500–3,000 ms with satisfactory results. Usually, a (time to echo) TE of 135–144 ms is used, as the metabolites usually found in hydatids are reasonably detected at these TE values, although some researchers have also used shorter TEs in addition (Chang et al. 1998). A variable NEX can be performed, depending on several factors. It is preferable to obtain the spectrum from the cystic part, avoiding the solid tissue/wall/host tissue as it can result in further metabolites (Fig. 13.3).
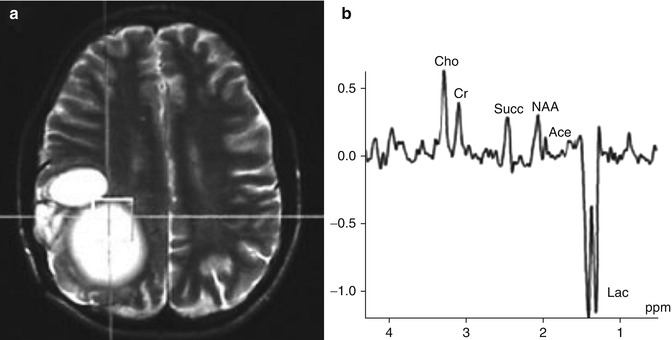

Fig. 13.3
In vivo 1H MRS, showing the voxel over hydatid cyst (a) and the spectrum obtained using a TE of 135 ms (b). The MRS in (b) shows a large inverted lactate peak (Lac), a prominent succinate (Succ) peak and a smaller acetate peak (Ace). Further choline (Cho), creatine (Cr) and NAA peaks are present due to contamination from the surrounding tissue (From Chand et al. (2005) with permission)
Typical Spectrum of In Vivo Proton Magnetic Resonance Spectroscopy in Hydatid Cysts
Of the several metabolites described, only a few are routinely picked up reliably on in vivo 1H MRS due to sensitivity issues. In particular the peak at 2.4 ppm is important as both pyruvate and succinate are detected at 2.4 ppm. These are difficult to differentiate on in vivo 1H MRS, and as discussed above, there is no consensus in the literature as to which of these metabolites are predominantly represented at 2.4 ppm, although their presence has been confirmed in separate studies using different techniques (Garg et al. 2002; Jayakumar et al. 2003, 2004). While this issue is pending, awaiting further investigation, still, a prominent peak at 2.4 ppm (pyruvate/succinate) is one of the most important features in in vivo 1H MRS.
Other important metabolites detected by in vivo technique are lactate (1.3 ppm, seen as a doublet), acetate (1.9 ppm) and alanine (1.5 ppm) (Chang et al. 1998; Garg et al. 2002; Jayakumar et al. 2003). While these could be variable, lactate is usually seen as a significant peak. Acetate is detectable although it is a smaller peak relative to the succinate/pyruvate. As a result, the ratio of succinate/acetate is generally quite high in hydatid cysts and is an important differentiating feature with pyogenic abscesses, where acetate/succinate ratio is high (Poptani et al. 1995; Martinez-Perez et al. 1997; Dev et al. 1998; Chand et al. 2005). It has been further suggested that various metabolites described in hydatid cysts may not be seen in all spectra, due to changes in internal milieu during evolution of the cyst (Jayakumar et al. 2003). Also, various forms of echinococcus may have different consumptions of oxygen and glycogen, thereby producing different concentration of metabolites (Jayakumar et al. 2003). Thus, despite having some uniformity in basic spectra, variations are possible.
Ex vivo 1H MRS detects a larger number of metabolites from hydatid fluid. These include amino acids leucine, valine and isoleucine (0.9–1.2 ppm), glycine (3.56 ppm), Cho (3.2 ppm), malate (4.3 ppm), fumarate (6.5 ppm), glucose and some of the aromatic amino acids (Garg et al. 2002).
In Vivo Proton Magnetic Resonance Spectroscopy in Other Cystic Conditions
Neurocysticercosis
The size and appearance of NCC are usually fairly different from hydatid cysts and rarely present a diagnostic dilemma; however, large and racemose lesions can create difficulty in diagnosis on routine MRI (Jayakumar et al. 2003). The issue becomes important due to the different management of these conditions (medical in NCC versus surgical in hydatid).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree