Fig. 26.1
Schematic image of sentinel lymph node (SLN) concept. SLN has direct flow from the tumor and the first metastasis via lymph channel should occur from here. If SLN harbors metastasis, second-tier node also has possibility to have metastasis. Inversely, if SLN shows no metastasis, no other lymph nodes in the regional basin will have metastasis
SLNB was initially performed using blue dye (BD) [7]. However, this technique has several limitations, such as a loss of visibility when lymphatic channels are in deep fat and rapid washout of dye from the dyed node [12, 13]. A significant technical learning curve for SLN biopsy has been reported, and substantial experience is required to develop the technical skill necessary to achieve a high success rate [12, 13]. To overcome these issues, BD combined with preoperative lymphoscintigraphy using radioisotope (RI) followed with an intraoperative handheld gamma-ray detector became the standard method to detect SLNs, providing a detection rate of nearly 98 % [14, 15]. Although the combined use of RI and BD affords a high SLN detection rate, 3–5 % of patients diagnosed as having negative SLN develop nodal metastasis (false-negative SLNB) [16, 17].
Kitai et al. [18] reported a new lymphatic mapping method using indocyanine green fluorescence imaging (ICG-FI) in breast cancer patients. Our attention was attracted by this ICG-FI because ICG fluorescence penetrates human tissue to a depth of 1–2 cm and we can transcutaneously detect the fluorescence in real time [19]. We started using this new ICG-FI combined with conventional RI and BD from 2010 [9]. After a series of cases in which ICG-FI was used, we became aware that some of the SLNs were detected only by ICG-FI. In this chapter, we would like to show our recent results obtained from SLNB using a combination of these three methods and to discuss the possibility of redetecting SLNs, which were overlooked by the conventional detection method.
Conventional Tracers for Sentinel Node Detection
Blue Dye Method
BD is safe, convenient, and cost-effective, but the detection rate was not satisfactory; BD by itself was reported to have a detection rate of 82 % [7]. As mentioned above, this technique has certain limitations, such as a loss of visibility in dense fat and rapid transit of the dye [12, 13]. However, BD is still useful in the operation because surgeons can confirm the blue lymphatic channel and lymph node by their own vision. Although a rare occurrence, we should remember possible allergic reaction to these dyes [20] and reduction in arterial oxygen saturation measured by pulse oximetry [21].
Radioisotope Method
To compensate for the limitations of BD, combining it with the use of radioisotope for tracers in detecting SLN is now a standard for SLNB. There are several different types of tracers depending on the conjugated particles. In general, 4–6 intradermal injections of 5–10 MBq of radiotracer are instilled closely around the tumor site. After injection, acquisition of dynamic images including the injection site to the regional lymph node basin continues until the RI drain into the SLN. This procedure is very important when using small-particle RI because they can easily pass through the SLN and accumulate at the second-tier nodes. At operation, the use of an intraoperative gamma-ray detector is required to search for “invisible” radioactive SLN. However, when the primary tumor is located near the regional lymphatic basin, the shine-through effect, which is a radiation from the injection site, interferes with the detection of SLN [22]. Thus, SLNB in such patients is sometimes difficult and leads to the failure to harvest SLN.
Indocyanine Green Fluorescence Imaging
Principle
ICG is widely used in a variety of examinations, including hepatic function, cardiac output, and retinal angiography [18], and is proven as a safe reagent. It binds with albumin and produces a peak wavelength of 840-nm near-infrared fluorescence when excited with 765-nm light (Fig. 26.2) [23]. This fluorescence is invisible to our eye, but a coupled-charged device (CCD) camera can visualize this fluorescence on a display. Unlike visible light, near-infrared light can penetrate human tissue and allows detection to a depth of 1–2 cm under the skin. Thus, we can observe real-time lymph flow under the skin.
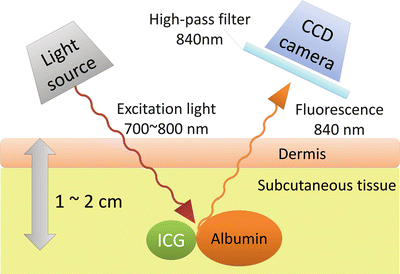

Fig. 26.2
Basic principle of indocyanine green fluorescence imaging (ICG-FI). The excitation wavelength of ICG that produces the maximum fluorescence is 765 nm, and the fluorescence occurs at 840 nm. This near-infrared fluorescence can be detected 1–2 cm beneath the skin
Required Instruments
The basic ICG fluorescence detection system costs about $50,000 USD in Japan. However, the principles of ICG-FI being very simple, we made our own detection system using commercially available parts which cost only $1,600 as described in the previous report [9]. Briefly, we used a light-emitting diode that generates 760-nm wavelength light as the excitation light and as a detector a CCD camera with a long-wavelength pass filter to filter out light with a wavelength below 840 nm. The fluorescence image is sent to a TV monitor for real-time monitoring via a video recorder. When in use, the main body of the system is covered with a sterilized vinyl bag, and we can use it at the operation site.
The basic system including our handmade detector can only display fluorescence as “white lesion” in a dark black and white image (Fig. 26.3a). This black and white image sometimes made it difficult to localize the small fluorescence in the operative fields. Recently, a new detector system, which can display fluorescence in a bright color image, has been introduced [24]. This new system may offer easier localization of fluorescence lymph channels and SLN, but it costs $120,000, about 70 times more than our handmade system [9, 24].
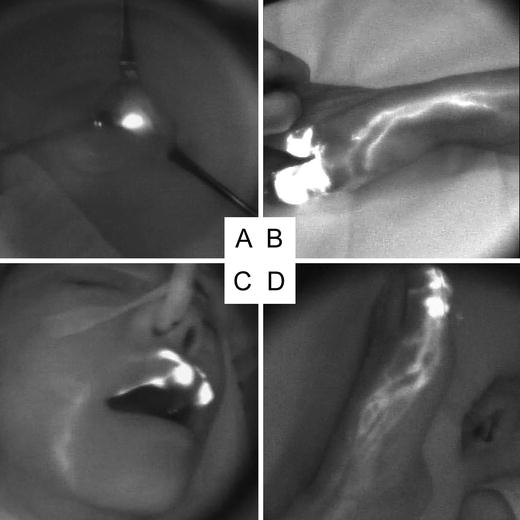

Fig. 26.3
Indocyanine green fluorescence images. (a) Lymph node stained with ICG displays as “white node”. (b) Acral lentiginous melanoma of the right thumb. Several lymph channels to the axillae were evident. (c) Lentigo maligna melanoma of the upper lip. Single lymph channel to the submandibular region was evident. (d) Acral lentiginous melanoma of the left great toe. Several lymph channels form a netlike network and run toward the groin
Injection
Dissolve ICG in physiologic saline at a concentration of 0.5 % solution. Inject ICG intradermally at the same spot where the RI is injected. From our experience, the sequence of injecting dye tracers, ICG and BD, does not affect SLN detection. Leaked ICG from the injection site should be wiped away using clean gauze, because ICG has very high fluorescence intensity and can easily contaminate the operation field.
Transcutaneous Localization of Sentinel Lymph Node(s)
Surgical light which includes wavelengths of 840 nm or longer should be turned off when detecting the fluorescence. However, there is no need to shut down all the lights in the operating room; the general ceiling lamp does not affect the observation of fluorescence. Soon after the injection of ICG, lymph flow from the injection site to the regional lymph node basin can be observed (Fig. 26.3b–d). The essential feature of this ICG-FI is real-time observation of lymph flow at operation; it enables us to see where the lymphatic channel is and localize the draining lymph node. In some cases, we can see the moving lymphatic fluid, which contains ICG moving toward the SLN. However, when a lymphatic channel is located under thick fat tissue or drains into solid tissue such as muscle or a salivary gland, transcutaneous observation of ICG fluorescence becomes difficult. In such cases, we can improve fluorescence observation by compressing the overlying skin and reducing the depth of fluorescence (Fig. 26.4a, b) [9, 25]. In our results, transcutaneous localization of SLNs by ICG-FI was possible for 139 of 190 SLNs (73 %).
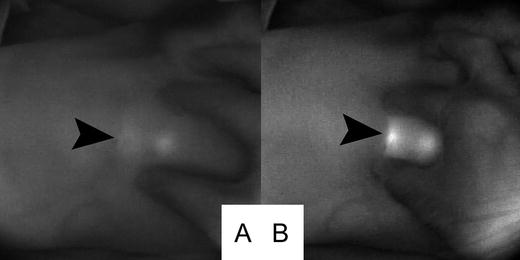

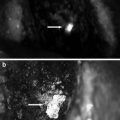
Fig. 26.4
Improvement of fluorescence detection by compression. Arrowhead indicates the location of the sentinel lymph node. (a) Fluorescence image before compression. (b) Improvement of fluorescence detection by compression
Visualization of Lymphatic Flow to SLN Located in Unusual Sites
It was interesting for us to see how the lymphatic channels connected to SLNs are located in unusual sites, such as cubital and popliteal lymph node basins. We experienced two patients with SLNs in cubital nodes and three patients with them in popliteal lymph nodes. The patients with SLN in the cubital region both had two main lymph channels on the radial side of the forearm and the channel, which runs outside and made a sudden curve towards the lateral side of the upper arm and finally drained into the cubital LN. The patients with SLNs in the popliteal region had completely different lymph channels which drain into the groin region. The lymph flow toward the groin LN runs along with the greater saphenous vein, but the lymph flow toward the popliteal LN runs along the dorsal side of the lower leg where the lesser saphenous vein is.
Limitation of Indocyanine Green Fluorescence Imaging
ICG-FI has several limitations: (1) The maximum depth of detection is 2 cm from the surface of the skin, (2) ICG shows very high fluorescence intensity and only a small leak of ICG can contaminate the surgical field, (3) quantitative evaluation of fluorescence is difficult at present, and (4) a halogen surgical lamp contains light with wavelengths over 840 nm and must be turned off during fluorescence detection. Most of these issues can be solved by combined use of RI; however, assuming an RI-free situation, we suggest the following solutions.
For issues (1) and (2), we advise the use of preoperative ultrasonography and marking of potential lymph nodes. This marking helps to estimate the possible SLN location when the lymphatic channel cannot be traced as far as the draining SLN through the skin. Thus, the incision line can be drawn at the estimated SLN position and the risk of damaging unnecessary lymph channels reduced. However, the location of SLNs in the neck region varies, and fluorescence is difficult to detect through the skin when the SLN is located in a salivary gland or under the muscle. Therefore, SLN mapping in the neck region still requires the RI method.
At present, (3) quantitative evaluation of fluorescence is difficult, and this means that we cannot exclude the possibility of harvesting second-tier nodes because of their high fluorescence intensity (Fig. 26.5). To exclude second-tier nodes, observation of the dynamic image soon after the injection is essential; trace the fluorescent lymph channels toward the regional lymph node basin and locate the SLN.
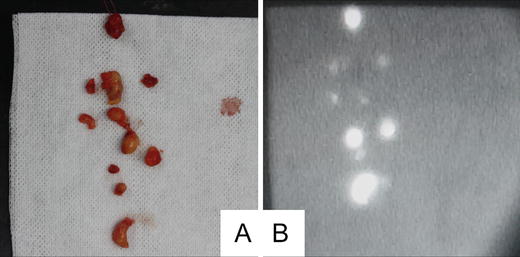

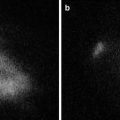
Fig. 26.5
Florescence detection in nonsentinel lymph nodes. (a) Photograph of dissected nonsentinel lymph nodes. (b) Fluorescence image of dissected nonsentinel lymph nodes. Most of the dissected nonsentinel lymph nodes showed fluorescence
With (4) the lighting issue, using a visible light source which does not contain 840-nm or longer wavelength is the best solution. Using a short-wavelength pass filter, which filters out long wavelengths from the conventional head light system, is one of the solutions [9]. Recently, surgical lighting systems are changing from using halogen lamps to light-emitting diodes (LED) because of their longer life, low energy consumption, and low generation of heat. LEDs produce light consisting of a very narrow range of wavelengths; therefore, it is theoretically possible to produce a surgical lighting system using LED which will not affect fluorescence imaging. Further development is required.
Comparison Between Indocyanine Green Fluorescence Imaging and the Conventional Detection Method in Skin Cancer: Experience with 76 Patients in Our Institute
Patients
We performed SLN biopsy using ICG-FI combined with the conventional methods from January 2010 through November 2013 on 75 patients with invasive skin cancer. Of these, 40 had malignant melanoma, 16 had squamous cell carcinoma, 15 had extramammary Paget’s disease, 1 had sweat gland carcinoma, and 1 had Merkel cell carcinoma, 1 had eccrine porocarcinoma, and 1 had clear cell sarcoma. Tumors were located in the head or neck in 13, upper extremity in 13, trunk in 30, and lower extremity in 19.
Procedures
Before the operation, lymphoscintigraphy was performed. On the morning of the operation, 99mTc-tin colloid was intradermally injected around the primary tumor. We marked the injected sites for the subsequent injections of ICG and BD. After the induction of general anesthesia, 0.4–0.8 mL of 2 % patent blue solution and 0.5 % ICG were intradermally injected at the marked spot around the primary tumor, and ultrasonography was performed to locate potential lymph nodes. Basically, the skin incision line was determined according to the lymphoscintigraphy results. For lymphatic basins in which only the ICG flow was confirmed, the skin incision line was determined according to the results of the ICG-FI with ultrasonography support. An intraoperative gamma-ray detector was also used to detect SLNs. The lymph nodes were considered to be SLNs and removed when any of the following conditions were observed: (1) lymph flow from the primary site to the draining lymphatic basin and a fluorescent lymph node, (2) a radioactive lymph node (including the hottest node and nodes that showed higher than 10 % of the maximum value as measured by the gamma probe), and (3) accumulation of BD in the lymph nodes. SLNs identified only by ICG-FI were termed “occult” SLNs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree