Infections
Nonsuppurative Infections
Blastomycosis
Background
Blastomycosis, also called Gilchrist disease or North American blastomycosis, is produced by the fungus Blastomyces dermatitidis.1,15 B. dermatitidis is inhaled into the lungs and causes pneumonitis.15,48 Although blastomycosis affects immunocompromised individuals, it can affect healthy individuals as well. Hematogenous spread may lead to involvement of the skin and osseous structures. The disease also can cause the patient to develop asymptomatic bone lesions.
Blastomycosis is endemic to the central and Great Lakes regions and the Ohio and Mississippi River valleys55 of the United States and to regions of Canada.1 Based on confirmed cases, the annual incidence is less than one case per 100,000 people in Mississippi, Kentucky, Arkansas, and Wisconsin.11,13,31 African Americans and Native Americans are more commonly affected than whites.55 Men are affected more often than women, with the ratio of men to women ranging from 4 : 1 to 15 : 1.55 Infections often develop in people who have contact with soil, work outside, or frequently engage in outdoor activities15,38,55; some cases of human–human infection transmission have been noted. Blastomycosis develops less frequently than other dimorphic fungi infections in immunocompromised individuals.15
Imaging Findings
Skeletal infections develop in up to 60% of blastomycosis cases, representing 25% of the extrapulmonary infections.1,15 Bone is the third most commonly infected site after the pulmonary system and skin38; in some cases, the disease may spread to the prostate.15 The vertebral bodies, ribs, and skull are the most commonly affected bones. Features of blastomycosis bone invasion include eccentric, well-circumscribed lesions that cause no significant periosteal reactions and have little cortical penetration.38 Punched-out lesions occasionally are reported, but sequestration rarely occurs.38 Although the vertebrae may be significantly damaged, the intervertebral discs may be spared. The infection may spread along the anterior longitudinal ligament and skip a vertebral level—a feature that also is associated with tuberculosis.55
Blastomycosis joint involvement is usually monoarticular; however, polyarticular presentations may be seen. Systemic or pulmonary disease may develop concurrently.15 The knee is most commonly infected, followed by the ankle, elbow, wrist, and hand.1,15 In some cases, blastomycosis presents as a soft-tissue mass that is solid, has punctate calcifications, and is similar to a synovial cell sarcoma.1 A soft-tissue neoplasm cannot be differentiated from this fungal lesion by plain film radiography.3,55 In addition, neither ultrasound, magnetic resonance imaging (MRI), nor bone scanning can properly diagnose blastomycosis. Accurate diagnosis is based on a combination of radiographic localization, biopsy, and culture, or by sputum microscopy in the case of pneumonitis.38,40,20
Clinical Comments
Signs and symptoms of blastomycosis include subclinical infection, and signs and symptoms similar to acute histoplasmosis; patients with pneumonitis may be asymptomatic in up to 50% of cases. Typical features include joint and muscle pain, a productive cough, and chest pain that resolves spontaneously.15 There may also be fever, weight loss, night sweats, and pleuritic pain.48 Patients with blastomycosis who have essentially normal laboratory profiles (e.g., normal complete blood count [CBC], erythrocyte sedimentation rate [ESR], and differential count) may have draining ulcers.55 Leukocytosis with a left shift may be evident in pneumonic cases.20 In certain cases, the hematocrit values may suggest chronic infection. No skin test exists for blastomycosis—biopsy and culture are the keys to the diagnosis. Silver methenamine is used to detect the fungus. The culture may take 1 to 2 weeks to grow.
Sputum microscopy is “simple and inexpensive” with a high diagnostic yield; although sensitivity is only 40% in patients with pneumonitis.11 Serologic studies using enzyme-linked immunoassy indicate that different genotypes of B. dematitidis exist. Historically, mortality rates in treated patients in whom the disease had disseminated were as high as 23%.55 Patients with untreated disease have an 80% mortality rate.1
Treatment protocols for blastomycosis include débridement and administration of oral imidazole, ketoconazole, itraconazole, or amphotericin B. A 6-month course of treatment is typical. Patients who take amphotericin B must be closely monitored1; side effects include anorexia, nausea and vomiting, fever with chills, anemia, and nephrotoxicity. In spite of the numerous side effects, the benefits of using amphotericin B outweigh the risks.
Coccidioidomycosis
Background
Coccidioidomycosis, which is also called valley fever and desert rheumatism,4,60 is a systemic infection caused by the soil fungus Coccidioides immitis. It is endemic to the southwestern United States, San Joaquin Valley, Mexico, and regions of Central and South America.4,6,24,28,53,59 The fungus spores typically are inhaled into the lungs, which are the primary site of infection.22,47 Many patients have mild or no symptoms, so the infection is found incidentally.32
Individuals who are older than 65 years of age or have acquired immunodeficiency syndrome (AIDS) are the primary populations at risk of infection.47 Other individuals at risk are those being treated with steroids or chemotherapy. Some individuals may be genetically predisposed to contracting the infection.29 Although dissemination is rare, an increased risk for spread of the disease exists among Filipinos, African Americans, Mexicans, pregnant women, children younger than 5 years of age, adults older than 50 years of age, and immunosuppressed individuals.15,22 The skin and subcutaneous tissues are the most common sites of dissemination from pulmonary disease. The next most common site is the mediastinum, followed by the skeletal system.22 The incidence of coccidioidomycosis has dramatically increased since 1991.29 Currently 50,000 patients a year are diagnosed with the infection.15
Imaging Findings
Skeletal lesions are seen in 10% to 50% of the cases in which the disease has spread. Coccidioidomycosis may affect multiple sites, producing osteolytic lesions in cancellous and cortical bone and soft-tissue swelling. A periosteal reaction is possible but uncommon.15,22,29 Previously, bony prominences were considered common sites for coccidioidomycosis infection. More recent studies have failed to document this association and have found that involvement of the axial skeletal is more common.15,32 In the appendicular skeleton, the ankles and knees are more frequently affected; remission occurs in 2 to 4 weeks, and no residual damage remains.15
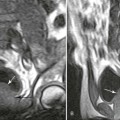
Plain film radiography may not detect infection during the initial stages. Findings of decreased joint space and areas of localized osteopenia may be early signs.15,32 Ankylosis can be an end result of joint infection.15 A bone scan is sensitive and typically can detect the infection in the early stages.22,32 MRI may demonstrate a large, poorly marginated mass with an intermediate signal and lower central signal. MRI also typically demonstrates a decreased signal on T1-weighted images and an increased signal on T2-weighted images, together a sign of abscess or necrosis.39 MRI and computed tomography (CT) are useful for detection and surgical planning.42 A positive bone scan should be followed by CT or MRI. A decreased disc space and gibbous formation may be seen, but are much less common than they are in patients with tuberculosis.22 CT demonstrates low-attenuation lesions that may appear bubbly and expansile.22
Septic arthritis secondary to coccidioidomycosis may develop and is usually seen as: (a) synovitis with joint effusion, (b) periarticular bone destruction similar to that in patients with tuberculosis or neuropathic joints, and (c) well-defined periarticular erosions resembling pigmented villonodular synovitis. Periosteal reactions typically are associated with a permeative destructive pattern.22 Soft-tissue swelling and osteoporosis also may be seen in conjunction with permeative destructive lesions. A periosteal reaction is not commonly associated with punched-out lesions. Some lesions demonstrate a thin, sclerotic margin surrounding the area of bone involvement that indicates reactive sclerosis.56
Clinical Comments
Coccidioidomycotic infection of bone and joints is uncommon; therefore, it is often overlooked as an initial diagnostic option, resulting in frequent delays in diagnosis.32 The clinical presentation closely mimics that of tuberculosis.5 Two-thirds of the patients recover uneventfully and may be asymptomatic throughout the course of their infection. One-third of patients develop symptoms that usually include a self-limiting pneumonitis caused by the inhaled airborne spores. Of those, 40% of patients present with the symptoms within 2 to 3 weeks of exposure. The signs and symptoms reported by 80% of the infected patients are fatigue, coughing, night sweats, and fever, a presentation that significantly resembles the flu.60 Pneumonia that does not respond to antibiotics may be part of an infected patient’s history.29 Other signs and symptoms are a general achiness, sore throat, peripheral eosinophilia, erythema nodosum, and erythema multiform.15 If arthralgia develops, it is usually polyarticular and migratory; tenderness and pain are common when the affected joint is moved through its range of motion, but effusion is not typical.15 In approximately 1% of the cases, the disease spreads to the skin, bones, joints, and lymph nodes, and, in rare cases, to the central nervous system.15,29,32,45 Spread of the disease has been reported to occur months to years after the primary infection.15
A brief visit to an area in which coccidioidomycosis is endemic is all that is needed to contract the infection. In one study, 24 of 25 patients with symptomatic coccidioidomycosis lived in endemic regions or were immunocompromised.32 Exposure and subsequent infection can begin insidiously.
An effective skin test can be used to detect previous coccidioidomycosis infection.29 A definitive diagnosis of an emerging case of coccidioidomycosis requires biopsy and culture.32 A tissue biopsy of an infected individual contains necrotic debris composed of polymorphonuclear leukocytes and often Langerhans cells. A culture takes 2 to 5 days to yield results. A microscopic examination is quicker but less sensitive.32 Methenamine silver is used during the laboratory examination to blacken the capsules of spherical fungi such as Coccidioides.5,29 Laboratory findings may include elevations in the white blood cell (WBC) count and ESR, but these results are not consistent.32
Surgical débridement is mandatory once the diagnosis of coccidioidomycosis in osseous or joint structures is made.32 Itraconazole is slow but effective, and fluconazole was found to be effective in nonmeningeal coccidioidomycosis. Amphotericin B should be reserved for severe infections with skeletal involvement.15 Open drainage, synovectomy, arthrodesis, and sometimes amputation are reserved for later stages of severe cases. If a timely diagnosis is made and proper treatment is instituted, patients with coccidioidomycosis have excellent outcomes, although fatalities do occur in rare instances of treated individuals.32
Maduromycosis (Mycetoma or Eumycetoma)
Background
Maduromycosis was first discovered in Madura, India, some time between 1842 and 1846.56 It predominantly affects the feet,36,52 which are infected by a penetrating trauma.62 Eumycetoma is a disease that is usually found in the tropics; only a few cases have been reported in temperate climates.36,53 The organism that causes the disease has been isolated in soil and on thorns, the most likely sources of infection. Mycetoma is produced by a number of organisms that are distributed in two distinct groups14: eumycetes, or true fungi, and actinomycetes, or false fungi, which is a filamentous bacterium. The organism that is usually responsible for maduromycosis in the United States and Canada is Petriellidum boydii; in Africa and India, it is Madurella mycetomi; in Mexico, it is Nocardia brasiliensis, and in Japan, it is Nocardia asteroides.14 Maduromycosis is rare in the United States; it is slightly more common in Mexico, Guatemala, and other parts of South America.52 Because pharmacologic treatments are frequently unsuccessful, surgical resection or amputation is often necessary.4
Imaging Findings
Radiographic characteristics of maduromycosis include mottled osseous destructive patterns with a periosteal reaction14,52 and marked soft-tissue swelling, often resulting in permanent deformities.52 When the periosteum is breached, a laminated, or spiculated, periosteal reaction is seen. The cortex often is eroded. Eumycotic lesions tend to be fewer in number and produce larger bone cavities that are greater than 1 cm, and a periosteal reaction occurs in approximately 50% of cases.36 Actinomycetes produce more numerous, smaller lesions, and a periosteal reaction is seen in approximately 75% of cases.36 In all cases, a soft-tissue mass is seen. The sequestrum associated with other types of osteomyelitis is not a feature of maduromycosis.14 Loss of cortical margins followed by erosion and adjacent sclerosis is more typical. If the infection develops near a joint, the cartilage is typically spared; however, ankylosis may develop as an end result of this process.14 Osteopenia is rare, possibly because patients can walk on even a severely infected foot, evidence that this type of infection typically is painless.36
Clinical Comments
General signs and symptoms of maduromycosis include the presence of multiple, crusted nodules surrounded by hyperpigmented tissue. The common clinical triad consists of a sinus tract, granules (colonies), and nodular enlargements of infected body parts.52 Hematologic laboratory values usually are normal. Patients with maduromycosis often have long histories associated with the development of the disease; one documented case lasted for 18 years.52
The disease is almost painless, and few constitutional symptoms are seen unless a secondary infection has developed.53 The bacteria initially invade soft tissue around superficial and deep fascia. The first sign typically is a small bump; the foot then becomes swollen (but not painful).14 The affected limb becomes deformed as a result of microbial colonies forming grains and intercommunicating sinuses that may drain onto the skin’s surface.36 The grains extrude and form colonies that vary in color according to the infecting species.36 The lesion is usually confined to the soft tissues for years before bone infection occurs.36 When the disease breaks into the bone in the later stages, it is usually painless. Hematogenous spread is rare.14
An antimicrobial treatment may be sufficient while the infection is limited to the soft tissues.36 Once the bones are involved, a cure may be impossible and amputation may be necessary, although it is considered to be a last resort.14,36 Differentiation between eumycetoma and an actinomycetoma is important because the most effective treatments for each type are different.53 Some actinomycetomas respond to high doses and long-term usage of antibiotics.14 In particular, Nocardia organisms seem to be sensitive to some of the sulfonamides. Use of solely surgical débridement and resection rarely cures the infection. Some recent progress has been made using itraconazole for 6 months.52
Syphilis
Background
Syphilis is a chronic systemic infection caused by the spirochete Treponema pallidum.54 Infections are capable of involving any organ or tissue and are classified as either congenital or acquired.50 Congenital syphilis is contracted by transplacental exposure of the fetus to the infection after about the fourth month of pregnancy. It is categorized into early and late presentations. Early congenital syphilis is diagnosed in children who are younger than 2 years of age, whereas late congenital syphilis is diagnosed in children who are 2 years and older.50 Acquired syphilis is contracted by close physical (usually sexual) contact with an infected individual’s skin lesions or mucous membranes. The disease initially develops as a chancre with local lymphadenopathy. The infection progresses over a period of several months and becomes systemic, or secondary, syphilis. After a latent period of 5 to 15 years, the disease progresses to the tertiary stage, which is characterized by gumma formation. Gummas are rubbery, soft, destructive lesions of various sizes that contain necrotic caseous material and affect bone, skin, viscera, the central nervous system, and other organs and tissues.
Although the incidence of syphilis decreased for many years after World War II, it increased through the 1970s and 1980s and peaked in 1988.9,25 It has been on the decline since 1997. The increase in the number of congenital syphilis cases has been attributed to an associated rise in the number of women with syphilis who subsequently became pregnant but did not receive adequate prenatal care.9 Drug addiction also has been associated with the increase, because individuals who abuse drugs have impaired immune systems and are less likely to take protective measures against sexually transmitted diseases while engaging in sexual activity.25 A clear association has been described between the use of crack cocaine and an increased incidence of syphilis.25 It was reported in 1988 that congenital syphilis had reached its highest levels since widespread use of penicillin began.54 The increase is particularly high among homosexual men. Many cases of congenital and especially acquired syphilis are never reported. It is likely that morbidity and mortality figures for both varieties are underestimated.54
Yaws is caused by another species of Treponema, Treponema pertenue, which is similar to T. pallidum. Yaws primarily develops in children, is an acquired disease through direct contact, but is rarely transmitted sexually. The radiographic findings are similar to those of syphilis, but symptoms tend to be more severe.
Imaging Findings
Congenital Syphilis.
Early.
Some radiographic evidence of the disease usually can be obtained from most symptomatic newborns with syphilis.9 Early manifestations of congenital syphilis include osteochondritis or metaphysitis, periostitis, and diaphyseal osteitis. Polyostotic, symmetric bone involvement is characteristic of congenital syphilis.23,50,57,65 The long bones, pelvis, vertebrae, and small tubular bones are involved.23
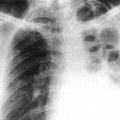
The first stage of osteochondritis, or metaphysitis, leads to fragmentation of the metaphysis, with transverse lucencies arising subjacent to the epiphyseal plate (Fig. 12-1). Invading granulation tissue is responsible for the metaphysitis57 and is common around the large joints, knees, shoulders, and elbows. Horizontal radiolucent metaphyseal bands are seen in the early stages of the disease. The ossification of the epiphyseal cartilage may be impeded, and a zone of rarefaction may be present at the epiphyseal line, indicating the presence of syphilitic granulation tissue.65 This tissue can weaken the bone and cause a pathologic fracture. Metaphyseal abnormalities are seen in more than 90% of infants with symptomatic congenital syphilis.9 The most common radiographic finding in patients with congenital syphilis is lucent metaphyseal bands.25
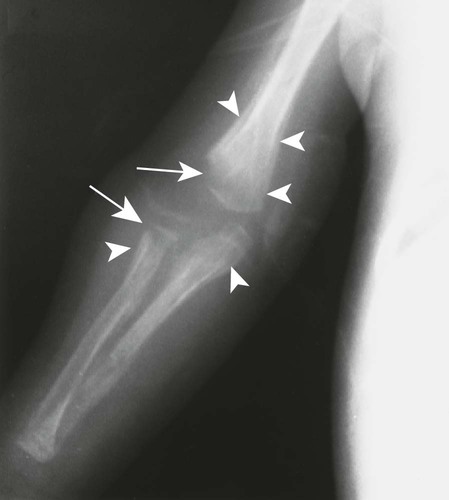
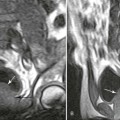
Metaphysitis results in erosive bone destruction, producing a characteristic sawtooth pattern of bone destruction. Although the sawtooth appearance of the metaphyseal margin is characteristic, it is generally uncommon.50 Radiographic examination of the destructive pattern of metaphysitis (which follows longitudinal bone growth) may reveal streaking radiolucent bands extending centrally from the physis, an appearance that resembles a celery stalk. Destructive metaphysitis is particularly common along the medial margin of the proximal tibia, a finding known as Wimberger sign (Fig. 12-2).50 Although this sign is a characteristic of syphilis, it is not pathognomonic.
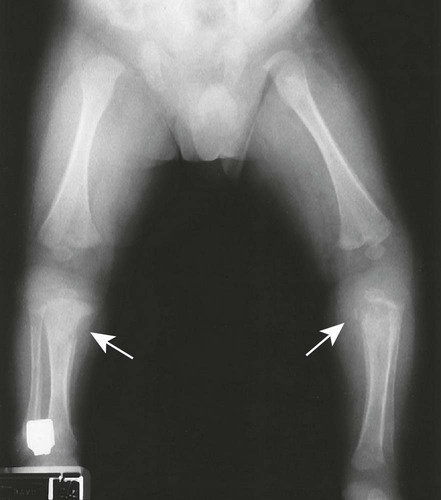
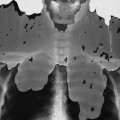
The second stage of early congenital syphilis is marked by bilateral, symmetric, diffuse periostitis affecting the long tubular bones but usually sparing the short tubular bones of the hands and feet.6,50,65 Although it is less common, a third stage may develop that exhibits radiolucent long bone defects consistent with osteitis. Osteitis is commonly associated with a diffuse region of the diaphysis that has a moth-eaten appearance.50 These changes can occur in any bone, but the femur and humerus are most commonly affected.65 Reparative periostitis during this phase may produce prolific changes along the anterior margin of the tibia, creating a saber shin deformity.
Late.
Late manifestations of congenital syphilis include destruction of and periostitis in the tibia, skull, jaw, nose, maxilla, and other superficial osseous structures. These changes are noted in patients in their late teens or early twenties. Periostitis is most pronounced along the anterior surface of the tibia bilaterally, creating a saber shin deformity. The destructive changes result from osteomyelitis, which may be associated with gumma formations. Clutton joints refer to the joints that undergo the particular pattern of destruction. The abnormally peg-shaped teeth secondary to syphilis are known as Hutchinson teeth.
Acquired Syphilis.
Radiographic findings are mostly confined to the tertiary stages of acquired syphilis. Less than 10% of patients with acquired syphilis ever develop an osseous lesion. The changes that do occur mimic those associated with late congenital syphilis. The most pronounced radiographic findings consist of proliferative and destructive changes affecting superficial osseous structures, particularly the tibia, clavicle, jaw, maxilla, and skull. It is extremely uncommon to encounter disseminated skeletal lesions in either infants or adults, and it is particularly rare to encounter them in patients who live in developed countries.64 The proliferative changes manifest as prolific periostitis especially prominent along the anterior surface of the tibia, producing bilateral anterior bowing or a saber shin deformity. Destructive changes are secondary to gumma formations that most commonly appear in the skull, nose, and diaphysis of long bones.
Clinical Comments
Congenital syphilis should be suspected in a newborn whose mother has positive serologic evidence of syphilis and is receiving inadequate or no treatment.25 Anemia, hepatosplenomegaly, skin lesions, and rhinitis are the predominant signs and symptoms of affected newborns. Some infants also have gastroenteritis, bronchopneumonia, septicemia, meningitis, pseudoparalysis, and joint swelling. A small number of children may have polydactylism, pathologic fractures, and signs and symptoms mimicking brachial plexus lesions.50 Thermal instability, mucocutaneous lesions, snuffles, hepatosplenomegaly, adenopathy, anemia, hydrops fetalis, pathological jaundice, and pseudoparalysis are consistent with syphilis.9
Babies with congenital syphilis are four times more likely to have low birth weights than babies without syphilis. Approximately 62% of patients with congenital syphilis are symptomatic at birth.57 Microhemagglutination tests usually are used to make a diagnosis,25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree