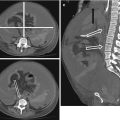
Fig. 15.1
Epidural collection in a 19-year old male. A lentiform collection crosses the midline, displacing the falx cerebri posteriorly. The collection is hyperintense on T2 (a), hypointense with thick peripheral enhancement on post-contrast T1 (b)
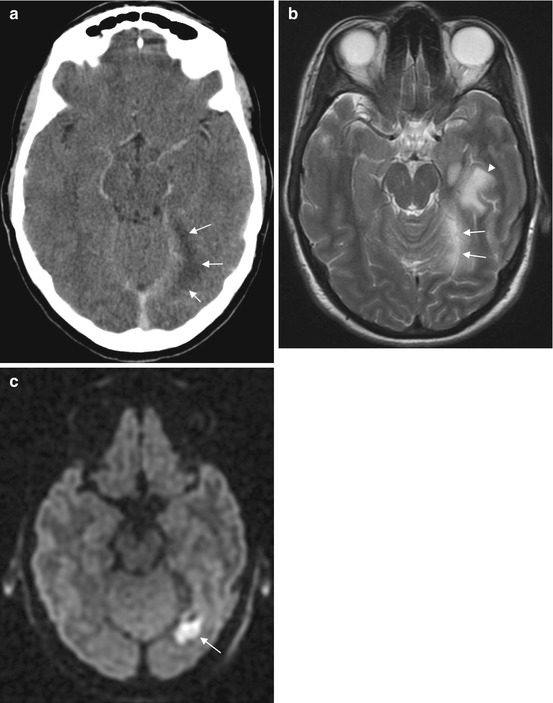
Fig. 15.2
Subdural empyema in a 34-year-old patient. The subdural empyema (arrows) is hypodense on CT (a), hyperintense on T2 (b) and hyperintense on diffusion-weighted imaging consistent with infection (c). The associated parenchymal T2 hyperintensity (b, arrowhead) is due to cerebritis
Both CT and MRI may demonstrate an associated cause such as sinonasal or mastoid opacification.
Meningitis
Meningitis has infectious and non-infectious causes. Infectious meningitis may be caused by a wide variety of organisms, most frequently through hematogenous spread. Other routes include direct extension from an extra-cranial source or rarely by direct inoculation following trauma. Non-infectious causes that should be considered comprise a wide differential including carcinomatosis, drug-induced meningitis, chemical meningitis, sarcoidosis and collagen vascular disorders. Given its rapid and fulminant course bacterial meningitis is the most likely cause in the critically ill patient.
The role of imaging in meningitis is not to make the initial diagnosis but to rule out mimics and assess for complications. Although imaging findings are not usually specific to a causative organism, imaging can be helpful to classify a detected lesion into infectious or inflammatory versus neoplastic disease.
Lumbar puncture (LP) should not be delayed until after imaging has taken place. Signs of possible raised intracranial pressure for which CT is recommended prior to LP are outlined in Table 15.1.
Table 15.1
Guidelines suggesting CT prior to LP to exclude imaging evidence of raised intracranial pressure
Infectious Diseases Society of America (IDSA) guidelines 2004 | National Institute for Health and Care Excellence Guidelines 2010 |
|---|---|
Immunocompromised state (eg, HIV infection, immunosuppressive therapy, solid organ or hematopoietic stem cell transplantation) | Reduced or fluctuating level of consciousness (Glasgow Coma Scale score less than 9 or a drop of 3 or more) |
History of CNS disease (mass lesion, stroke, or focal infection) | Relative bradycardia and hypertension |
Abnormal level of consciousness | Abnormal posture or posturing |
Focal neurologic deficit | Focal neurological signs |
Papilloedema | Papilloedema, abnormal ‘doll’s eye’ movements |
New onset seizure (within 1 week of presentation) | Unequal, dilated or poorly responsive pupils |
MRI is more sensitive to meningeal enhancement than CT. In the early stages of the disease, both CT and MRI may be normal. The hallmark of meningitis from any cause is contrast enhancement of the meninges, which can be classified into two patterns:
Leptomeningeal enhancement of the pia-arachnoid layers, with a serpentine or gyriform appearance that follows the pial surface of the brain, filling the subarachnoid spaces of sulci and cisterns
Pachymeningeal enhancement of the dura-arachnoid layers, parallel to the calvarium, or of dural reflections (falx cerebri, falx cerebelli, tentorium cerebelli and cavernous sinus).
Infectious meningitis is generally associated with leptomeningeal enhancement (Fig. 15.3a–c). Enhancement from viral and bacterial meningitis is typically thin and linear, whilst fungal meningitis tends to be nodular and thicker. Cranial nerve enhancement, which is always abnormal, can be associated with viral meningitis or neoplastic disease such as lymphoma and leukemia.
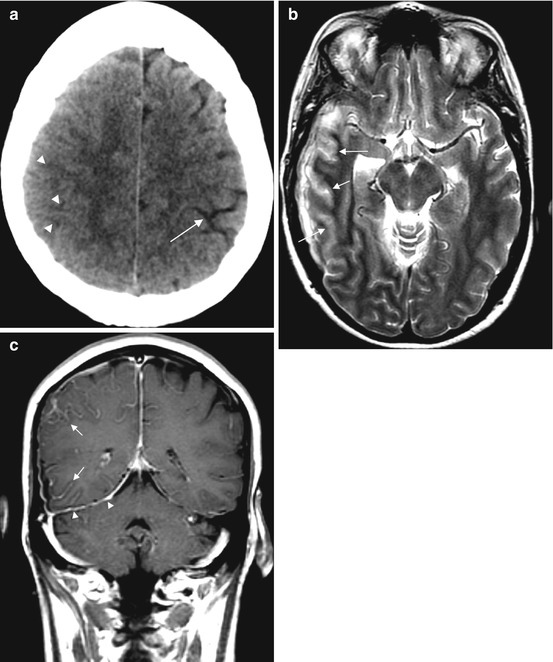

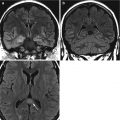
Fig. 15.3
Meningitis in a 50-year-old patient. CT (a) demonstrates subtle sulcal effacement (arrowheads) on the right compared to the left (arrow). The T2-weighted image (b) demonstrates cortical swelling and hyperintensity in the affected lobes (arrows). The T1 post-contrast image (c) shows leptomeningeal enhancement (arrows) and pachymeningeal enhancement (arrowheads)
Pachymeningeal enhancement is seen transiently in post-operative patients, secondary to intracranial hypotension (rarely following lumbar puncture), or due to granulomatous disease or malignancy such as metastatic breast and prostate cancer and secondary CNS lymphoma.
Thick leptomeningeal enhancement in the basal cisterns, as opposed to leptomeningeal enhancement in the convexities, is more likely seen in chronic meningitides such as tuberculous or fungal meningitis (Fig. 15.4). This is thought to be due to a thick, gelatinous exudate found in the basal cisterns. It is also demonstrated as hyperdensity in the basal cisterns on unenhanced CT. The triad of basal meningeal enhancement plus the associated complications of hydrocephalus and infarcts is specific for tuberculous meningitis.
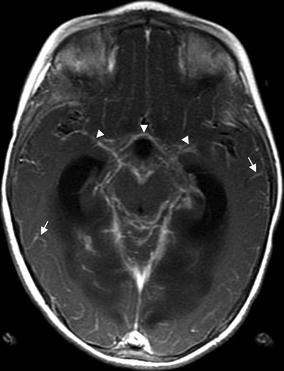

Fig. 15.4
Basilar meningitis in TB, extensive leptomeningeal enhancement in a 4-year-old child with tuberculous meningitis. The T1 post-contrast images show thick leptomeningeal enhancement around the basal cisterns and Sylvian fissures (arrowheads) and leptomeningeal enhancement in the convexity sulci throughout the brain (arrows)
Complications of Meningitis
Up to 50 % of adults with bacterial meningitis develop complications such as hydrocephalus, subdural effusions, subdural/epidural empyema, cerebritis, abscess formation and arteriopathy or venous thrombosis with associated infarction. Cerebrovascular complications are common, occurring in approximately one third of patients.
A hydrocephalus may be communicating, due to reduced absorption of CSF in meningitis or obstructive, due to the formation of adhesions and loculations, particularly in the cerebral aqueduct. Both CT and MRI are sensitive to changes in the size of the ventricles, best seen at the temporal horns or third ventricle.
Basal meningitis can lead to spasm or occlusion of small perforating vessels with subsequent infarction of the basal ganglia, or of large arteries, causing large cortical/subcortical infarcts (see also Chap. 14).
Cortical infarction disrupts the pia, a normal barrier to the spread of infection, enabling the development of cerebritis and abscess formation. Infection may also spread via retrograde thrombophlebitis. Cerebritis can be difficult to differentiate from infarction and a focus of infarction can develop into cerebritis. However, the two can be distinguished with time as cerebritis evolves into an abscess, whilst signal change from infarction persists in the affected vascular territory.
Ring-Enhancing Lesions
The spectrum of differential diagnoses for ring-enhancing lesions in the brain is enormous. It includes abscesses, tumors and primary CNS lymphoma, radiation necrosis, subacute infarction or hematoma and atypical infections such as tuberculosis, fungal or parasitic infections. Atypical infections are an important consideration in the immunocompromised patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree