(1)
Department of Clinical Radiology, Amiri Hospital – Kuwait City, Kuwait City, Kuwait
11.1 Fever
11.2 Giardiasis
11.3 Amebiasis
11.3.1 Intestinal Amebiasis
11.3.2 Hepatic Amebiasis
11.3.3 Thoracic Amebiasis
11.3.4 Brain Amebiasis
11.4.1 Skin Involvement
11.4.2 Nerve Involvement
11.4.3 Eye Involvement
11.4.4 Mucosal Involvement
11.4.5 Bone Involvement
11.4.6 Post-therapy Leprosy
11.5 Toxoplasmosis
11.7 Neurocysticercosis
11.8 Ascariasis
11.10.1 Echinococcus granulosus Disease
11.10.4 Echinococcus alveolaris Disease
11.11.1 Acute Chagas’ Disease
11.11.2 Subacute Chagas’ Disease
11.11.3 Latent Chagas’ Disease
11.11.4 Chronic Chagas’ Disease
11.12.1 Schistosoma Life Cycle
11.12.2 Schistosomiasis by S. japonicum
11.12.3 Schistosomiasis by S. mansoni
11.13 Tuberculosis
11.13.1 Pulmonary TB
11.13.2 Pleural TB
11.13.3 Miliary TB
11.13.4 Abdominal TB
11.13.5 Hepatic TB
11.13.6 Genitourinary TB
11.13.7 Musculoskeletal TB
11.13.8 Tuberculous Lymphadenitis
11.13.9 Dermatological TB
11.15 Malaria
11.16 Animal Bites and Stings
11.16.1 Rabies
11.16.2 Viper Bite
11.16.4 Hymenoptera Stings
11.17 Filariasis
11.19 Fever of Unknown Origin
11.1 Fever
Fever is a condition characterized by elevation of body temperature above the normal daily variation, along with an increase at the hypothalamic thermal set point. A substance that induces fever is called a pyrogen.
Some infections produce exogenous toxins that induce the synthesis of endogenous pyrogenic cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor. These endogenous pyrogens induce the synthesis of prostaglandin E2 (PGE2), which elevates the hypothalamic core temperature set point. When the hypothalamic set point is raised, vasoconstriction occurs, decreasing heat loss from the skin.
Fever can be caused by infections (e.g., abscess and septicemia), neoplasms (e.g., lymphoma), inflammatory diseases (e.g., collagen vascular disease), and other causes (e.g., Kawasaki syndrome).
Pyrexia of unknown origin (PUO) is defined as an illness of more than 3 weeks’ duration, fever >38.3 °C on three occasions, and necessary initial investigations that fail to reveal the cause of the fever. The necessary initial investigations include detailed history and physical examination, complete blood count, antinuclear antibodies, rheumatic factor, urinalysis, three blood cultures, urine culture, chest radiograph, abdominal sonography, and tuberculin skin test. In 25–35 % of PUO patients, a diagnosis cannot be made.
Central fever is a term used to describe fever that arises after intracerebral hemorrhage, in the absence of infection, inflammation, or a tumor explaining the fever. This fever has been attributed to cytokine-related elevation of the hypothalamic set point.
Signs on CT and MRI
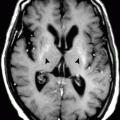
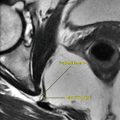
In patients with suspected central fever, the examination classically shows bleeding into the thalamus and the hypothalamus (Fig. 11.1.1).


Fig. 11.1.1
Axial CT (a), MR-T1W (b), and MR-T2W (c) images of a 26-year-old patient with intracranial bleeding after a car accident show multiple foci of intracranial bleeding. The patient developed high-grade fever, with no signs of infection detected in the serum or the cerebrospinal fluid. The scans showed focal intracranial hemorrhage involving the right lentiform nucleus (arrowhead) and the left thalamus (arrow). The patient was finally diagnosed as having central fever and was managed accordingly
What is the difference between fever and hyperthermia?
Hyperthermia is a condition characterized by uncontrolled increase in body temperature that exceeds the body’s ability to lose heat, in conjunction with a normal hypothalamic thermal set point.
Hyperthermia does not involve pyrogens and does not respond to antipyretics.
Further Reading
Chantal PBR, et al. Fever. Medicine. 2009a;37(1):28–34.
Chantal PBR, et al. Pyrexia of unknown origin. Medicine. 2005;33(3):33–6.
Deogaonkar A, et al. Fever is associated with third ventricle shift after intracerebral hemorrhage: pathophysiological implications. Neurol India. 2005;53(2):202–7.
Rudd P. Pyrexia of unknown origin (PUO). Curr Paediatr. 1996;6:105–7.
11.2 Giardiasis
Giardiasis is an infectious disease inducing fatty diarrhea caused by the intestinal protozoa Giardia lamblia. Protozoa are single-celled living organisms with two cell layers: an outer layer (ectoplasm) and an inner layer (endoplasm). In cases of environmental changes, the protozoa secrete a protective coat and shrink into a round, armored, infectious form called a “cyst.” When humans ingest the cyst, it transforms again into the motile form and is called “trophozoite.”
Giardiasis outbreak often occurs after sewage contamination of drinking water or after drinking from clear mountain streams contaminated with G. lamblia. After ingestion of the cysts, the parasites transform into trophozoites in the duodenum and jejunum and adhere to the intestinal wall. The parasites coat the duodenal and jejunal walls and interfere with fat absorption from the gut, resulting in fatty diarrhea. The parasites do not invade the intestinal wall, only coat it. The ileum is rarely affected by giardiasis.
Most patients with giardiasis are asymptomatic. Children and patients with low immunity may show mild abdominal discomfort, along with fatty diarrhea resembling celiac sprue or celiac disease diarrhea. Rarely, G. lamblia may invade the gallbladder, causing cholecystitis and jaundice. A concomitant infection with Entamoeba histolytica may be overlooked in cases of infection by a large number of G. lamblia.
There is an increased incidence of giardiasis in patients with hypogammaglobulinemia. Intestinal lymphoid hyperplasia (Peyer’s patches hyperplasia) may be found in cases of giardiasis infecting a patient with hypogammaglobulinemia. Diagnosis of giardiasis is confirmed by identifying the cysts in the stool.
Differential Diagnoses and Related Diseases
Gay bowel syndrome: there is an increased incidence of giardiasis in male homosexuals with diarrhea. Active male homosexuals may show cysts in the stool in up to 20 % of cases.
Signs on Barium Enteroclysis
As giardiasis mainly affects the duodenum and jejunum, bowel fold thickening, edema, and barium filling defects are usually seen in the duodenum and jejunum. Occasional barium segmentation or fragmentation may be seen (Fig. 11.2.1).


Fig. 11.2.1
Barium enteroclysis examination in a patient with giardiasis shows some irregularity and mucosal thickening of the second part of the duodenum. The proximal jejunum loops show edema, irritability, and poor filling, with thickening and separation of mucosal folds
Further Reading
David BH, et al. An update review on Cryptosporidium and Giardia. Gastroenterol Clin N Am. 2006;35:291–314.
Heymans HSA, et al. Giardiasis in childhood: an unnecessarily expensive diagnosis. Eur J Pediatr. 1987;146:401–3.
Maurice MR. Radiological diagnosis of giardiasis. Semin Roentgenol. 1997;32(4):291–300.
11.3 Amebiasis
Amebiasis is a parasitic infectious disease caused by the protozoan Entamoeba histolytica. It is the second most common cause of deaths from parasitic diseases after malaria.
Amebiasis is endemic in Mexico, India, Central and South America, and East and South Africa. There is a high incidence of amebiasis among homosexual patients.
The infectious form of the parasite is the “mature cyst,” which is resistant to the gastric and gastrointestinal (GI) secretions. The mature cyst can survive harsh environmental conditions and is resistant to the conventional chlorine used to purify drinking water.
Amebiasis is initiated by ingestion of the mature cyst from infected water or food. The disease is often asymptomatic; however, multiple manifestations may be seen throughout the body, including bloody diarrhea in a small percentage of patients. Diagnosis is confirmed by identification of the amebic trophozoites in the stool or by serological identification of ameba-specific antibodies, usually 7 days after the initial symptoms of amebiasis.
Intestinal Amebiasis
Amebic invasion of the GI tract can result in different manifestations, each with its own radiological imaging features:
Ulcerative amebic rectocolitis (ambulatory dysentery): in 10 % of children, the trophozoites invade and penetrate the intestinal mucosa, resulting in intestinal erosions and bleeding. This is usually manifested as 4–6 episodes of bloody diarrhea per day without systemic manifestations or fever. Complications of ambulatory dysentery include anemia due to bloody diarrhea, intussusception, and/or rectal prolapse due to high-speed peristalsis.
Ameboma: a granulomatous colonic lesion of ameba, with necrosis, edema, and inflammation, resembling a pseudotumor superimposed by secondary infection. It arises from the cecum or the ascending colon wall, measuring 5–30 cm. Ameboma is often solitary but can be multiple. Patients complain of bloody diarrhea, abdominal pain, and a colonic mass.
Amebic appendicitis: cannot be differentiated from classic appendicitis, unless the patient’s history of bloody diarrhea is known.
Fulminant colitis with toxic megacolon: a rapidly progressing disease with up to 20 episodes of bloody diarrhea within 24 h. Patients present with abdominal pain, anorexia, fever, rapid pulse, hypovolemia, and intense, constant tenesmus. There is a high mortality rate, especially when massive thrombosis of the colonic wall venules and intestinal ischemia develop.
Chronic amebic colitis: this term is used to describe patients with chronic, nonspecific abdominal pain with occasional E. histolytica evidence in the stool.
Signs on Plain Abdominal Radiograph
Abdominal radiograph may show a dilated colon with loss of the haustrations (0.5 % of cases) (Fig. 11.3.1).


Fig. 11.3.1
A plain abdominal radiograph of a patient with chronic amebic dysentery shows toxic colonic dilatation of the ascending and the transverse colon (arrowhead)
Signs on Barium Enema
Ulcers: seen as fine granular appearance of the mucosa with margin speculations. Deep ulcers penetrate the mucosa and result in “collar-button” ulcers.
Thumbprinting: a term used to describe the shape of barium distribution inside the intestinal or colonic lumen due to edema. The barium will show filling defects at the mucosal edges as if a person’s thumb has erased the barium from the edges (Fig. 11.3.2).

Fig. 11.3.2
Intestinal barium CGI shows thumbprinting appearance (arrowheads)
Conical cecum: a term used to describe a rigid, ulcerated, and conical-shaped cecum. Conical cecum is often seen in intestinal tuberculosis, Crohn’s disease, and amebiasis, because the cecum is affected in 90 % of cases (Fig. 11.3.3).

Fig. 11.3.3
Intestinal barium CGI shows the classical appearance of conical cecum (arrowheads)
Ameboma: seen as barium filling defect that resembles a carcinoma.
Signs on CT
Colitis is seen as marked irregular thickening of the colonic wall, with enhancement after contrast administration.
Hepatic Amebiasis
Occasionally, the amebic trophozoites can reach the liver via the portal system and form an abscess (30 % of cases). GI invasion is often seen in children, with a mortality rate of 1 %, while the formation of liver abscess is often seen in adults, with a mortality rate of 0.2–2 %.
Hepatic abscess may not be preceded by a history of diarrhea (59 % of cases). Patients often present with sudden onset of right hypochondriac pain radiating to the shoulder or the subscapular area. There is associated fever, anorexia, and vomiting, and the pain is exacerbated by deep inspiration or sitting in the right lateral decubitus position. The abscess is often seen in the right lobe of the liver. Differentiation between amebic and pyogenic hepatic abscess is important for proper patient management.
Signs on Plain Chest Radiograph
Hepatic abscess may often reveal itself in the form of a raised right hemidiaphragm (Fig. 11.3.4).


Fig. 11.3.4
A plain chest radiograph shows a raised right diaphragm due to hepatic abscess
Signs on US
Abscesses are seen as masses of heterogeneous echogenicity, with irregular wall and poor peripheral definition. Internal fluid-fluid level might be seen.
Signs on CT
Hepatic abscesses can be either pyogenic (bacterial) or amebic (parasitic) in origin. It is often difficult to differentiate between amebic and pyogenic liver abscesses based on CT appearance alone, but some radiological clues may be of use:
Pyogenic abscess can present without any signs of infection and shows uniform ring enhancement after contrast injection, air fluid level may be seen inside the abscess, and it shows microabscesses (satellite lesions), which are occasionally seen as hypodense lesions >2 cm around the main abscess. A pyogenic abscess classically reveals yellowish fluid after aspiration.
Amebic abscess characteristically shows a halo of hypodensity surrounding the enhanced ring of the abscess, due to peripheral edema (Fig. 11.3.5). After aspiration, an amebic abscess classically reveals brown fluid (anchovy sauce appearance), although it may be a pyogenic abscess mixed with hemorrhage from the needle. An acute amebic abscess can transform into a chronic abscess, which is characterized by fibrosis and hard mass formation that can be mistaken for hepatocellular carcinoma.

Fig. 11.3.5
Axial hepatic postcontrast-enhanced CT images in two different patients with hepatic abscesses. In one patient (a), an amebic liver abscess is illustrated with its characteristic halo (arrowhead). Notice the multiple splenic microabscesses. In the other patient (b), a pyogenic liver abscess is demonstrated for comparison. Notice the lack of the surrounding halo, with the presence of multiple small satellite lesions (arrows)
Differential Diagnoses and Related Diseases
Meleney’s synergistic gangrene is a rare complication of progressive postoperative gangrene, which arises after empyema or intraperitoneal abscess drainage. This complication can arise due to Staphylococcus aureus infection or cutaneous amebiasis. Patients often present 10–14 days post empyema or intraperitoneal abscess drainage, with truncal ulcer and severe pain. Pathologically, the ulcer is sharply demarcated, with three zones of colors: an outer bright red zone, an inner raised purple zone, and a central zone of red granulation tissue obscured by yellowish exudates.
Thoracic Amebiasis
Amebiasis from liver abscess may extend to the right lower lung lobes via invading the diaphragm. This rare complication is often seen in adults. Thoracic amebiasis is almost always secondary to amebic hepatic abscess. Empyema, pericarditis, and mediastinitis may occur due to amebic extension into the thoracic structures.
Signs on Plain Chest Radiograph
Chest radiograph often shows a raised right hemidiaphragm along with right basal pleural effusion, pneumonic patch, or atelectasis.
Brain Amebiasis
Brain amebiasis is a rare complication seen in 1 % of patients with amebic dysentery. The parasites often reach the brain via the hematogenous route. Patients often present with convulsions, hemiplegia, meningitis, or cranial nerve lesions.
Signs on Brain CT
Amebic abscess is seen as a nonspecific parenchymal hypodense area with peripheral, uniform ring enhancement.
Further Reading
Avron B, et al. Biochemistry of Entamoeba: a review. Cell Biochem Funct. 1988;6:71–86.
Cade D, et al. Amoebic perforation of the intestine in children. Br J Surg. 1974;61:159–61.
Davson J, et al. Diagnosis of Meleney’s synergistic gangrene. Br J Surg. 1988;75:267–71.
Kimura K, et al. Amebiasis: modern diagnostic imaging with pathological and clinical correlation. Semin Roentgenol. 1997;32(4):250–75.
Yin LS, et al. Left lobe amoebic liver abscess mimicking a perforated gastric tumor. Eur J Radiol Extra. 2008;66:e25–7.
11.4 Leprosy (Hansen Disease)
Leprosy is a chronic, granulomatous, infectious disease that mainly affects the skin and the peripheral nerves and is caused by acid- and alcohol-fast bacilli Mycobacterium leprae (M. leprae).
Leprosy is divided into different clinical subtypes based on the capacity of the patient’s immune system to resist the disease:
Indeterminate leprosy: the initial form that either resolves spontaneously or progresses into the other forms according to the degree of cell-mediated immunity.
Tuberculoid leprosy (TL): a form of leprosy that results from strong cell-mediated immune response to the disease. This type is characterized by the formation of multiple skin and nerve granulomas (tubercles), often restricted to a few locations. The granulomatous reaction in TL mimics tuberculosis but is non-caseating.
Borderline leprosy: this form represents an intermediate state between the tuberculoid and the lepromatous forms of leprosy.
Lepromatous leprosy (LL): this form results from low immunity of the infected host, resulting in widespread, extensive disease damage. The granulomatous reaction in LL is characterized by the formation of “lepromas,” which are granuloma formations mediated by macrophages engulfing live bacteria (lepra cells).
The clinical features of leprosy are determined by the host response to M. leprae. Skin, nerves, eyes, mucosa, and bone may all be affected by leprosy. Laboratory results often show high erythrocyte sedimentation rate and increased finding of rheumatoid factor in LL (rheumatoid factor is positive in 58 % of cases).
Diagnosis of leprosy is established via clinical examination and identification of the acid- and alcohol-fast bacilli on skin or buccal mucosa smears stained by the Ziehl–Neelsen technique. Clinical diagnostic features of leprosy include skin lesions with definite sensory loss and thickened peripheral nerves.
Skin Involvement
In TL, the early skin manifestation is hypopigmented macules or plaques. A macule is a localized area with textural or color change of the skin, while a plaque is a palpable, plateau-like elevation of skin >2 cm in size. TL is characterized by few skin lesions, which are often dry, scaly, and hairless.
In LL, widespread symmetrically distributed macules are often seen as early skin changes. The macules are poorly defined and show erythema (redness due to vascular dilatation). If the macules are untreated, dermal infiltration occurs, which causes skin thickening. When skin thickness occurs in the face, it is called leonine facies (Fig. 11.4.1). The eyebrows and the eyelashes may be lost (madarosis). Leprous alopecia is characterized by scalp hair loss with preservation of the hair over the course of scalp arteries. Dystrophic nail changes, with development of peripheral edema of the legs and ankles, often occur. Souza Campos nodule is a rare form of TL seen in endemic areas of Brazil, where leprosy infection may infect patients during a kiss, and the highly resistant child develops a nodule at the site of the inoculation. Lucio phenomenon is a very rare reactional state of LL characterized by painful irregular skin patches that become purpuric and form bullae that break down, leaving widespread areas of ulceration. Healing is with scar formation. Lucio phenomenon arises due to cutaneous vasculitis.


Fig. 11.4.1
Forehead skin thickening and plaques (leonine facies) with loss of the eyebrows (madarosis)
Nerve Involvement
Nerve involvement in leprosy affects sensory, motor, and autonomic peripheral nerves. The posterior tibial nerve is mostly affected, resulting in anesthesia of the soles and feet. Involvement of the peripheral autonomic fibers results in loss of skin sweating, with glove-and-stocking hypohidrosis, a situation similar to the changes seen in diabetic peripheral neuropathy. Loss of peripheral joint sensation causes repetitive trauma and osteomyelitis, later resulting in the development of Charcot’s joint. Motor denervation of the hand causes progressive hand contracture deformity, known as “claw hand” (Fig. 11.4.2).


Fig. 11.4.2
Bilateral claw-hand deformities of leprosy
Eye Involvement
Blindness may occur in up to 5.3 % of patients with leprosy, due to an inability to close the eyes normally (lagophthalmos), corneal ulceration, secondary cataract, and chronic iridocyclitis.
Mucosal Involvement
Both the nasal and the oral mucosa may be affected by LL. Nasal mucosa involvement results in sneezing blood (epistaxis) due to ulceration and nasal stuffiness due to formation of polyps.
The oral mucosa is affected in TL and LL patients, who are often neglected and who receive delayed treatment. Oral mucosal lesions in LL include yellowish-white plaques, nodular infiltration of the tonsils, deep ulceration of the soft palate, and elongation of the uvula (Fig. 11.4.3).


Fig. 11.4.3
Oral mucosal involvement in leprosy. There is deep ulceration of the soft palate, tonsillar abscess formation, and elongation of the uvula
Bone Involvement
Bone involvement in leprosy may be seen due to reactional state arthritis (explained later), development of Charcot’s joint due to peripheral neuropathy, osteomyelitis, or due to destruction of facial bones by leprosy. In osteomyelitis, the bacteria directly invade the articular bone and its bone marrow, causing infection and destruction of the joint. Up to 80 % of bone changes in leprosy are seen in the hands and feet.
Facies leprosa describes a triad of facial skull lesions that has been used by paleopathologists as a reliable marker for leprosy in ancient skeletal remains. The triad includes atrophy of the anterior nasal spine, atrophy and recession of the alveolar processes of the maxillae, and endonasal inflammatory changes (Fig. 11.4.4).


Fig. 11.4.4
Bony changes of facies leprosa. Notice the destruction of the nasal spine and the recession of the alveolar processes of the maxillae
Post-therapy Leprosy
When therapy is started to eliminate M. leprae, a cell-mediated autoimmunity may result which starts to fight both the bacteria and the normal cells, known as reactional states. Reactional states may also occur spontaneously, without treatment. The eyes, skin, nerves, and ears become swollen and painful. Reactional states are mainly divided into two types:
Type 1 (reversal) reaction: this type of reaction is seen in patients with BL (30 % of cases) and is characterized by skin and nerve inflammation, edema, and ulceration.
Type 2 reaction (erythema nodosum leprosum): this type of reaction is seen mainly in LL (20 %) and TL (10 %). Symmetrical joint arthritis that involves small or large joints is a characteristic feature of patients with erythema nodosum leprosum (ENL). The arthritis can be monoarticular, oligoarticular, or polyarticular. The arthritis can be infective or sterile (reactive) arthritis. Reactive arthritis in leprosy has almost the typical clinical presentation as rheumatoid arthritis. In general, the severity of the arthritis parallels the severity of the ENL.
Signs on Radiographs
In joint osteomyelitis, there is destruction of the juxta-articular bone, with joint collapse (Fig. 11.4.6).
Diffuse osteoporosis.
Joint destruction due to neuropathic arthropathy (Charcot’s joint) (Fig. 11.4.5).

Fig. 11.4.5
A lateral ankle radiograph of a patient with leprosy shows severe calcaneus destruction
The phalanges often show thinning of the bone with reduction in thickness, a finding that is usually called “sucked lollipop appearance” (Fig. 11.4.6).

Fig. 11.4.6
An anteroposterior radiograph of the forefoot of the other foot of the same patient shows acroosteolysis of the distal phalanges (white arrowheads), fracture of the second metatarsal diaphysis (black arrowhead), sucked lollipop appearance of the fourth phalange (open arrowhead), and cortical destruction of the fourth metatarsal head due to osteomyelitis (arrow)
Osteitis leprosum are localized cortical bone erosions of the phalanges and metacarpals.
Acroosteolysis (bone resorption) of the terminal phalangeal tufts (Fig. 11.4.6).
Contracture of the hands, with soft-tissue swelling (claw-hand deformity).
Further Reading
Boddingius J. Ultrastructural and histopathological studies on the blood-nerve barrier and perineural barrier in leprosy neuropathy. Acta Neuropathol. 1984;64:282–96.
David MS, et al. Oropharyngeal leprosy in art, history, and medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:463–70.
de Abreu MAMM, et al. The oral mucosa in paucibacillary leprosy: a clinical and histopathological study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e48–52.
Gibson T. Bacterial infections: the arthritis of leprosy. Baillière’s Clin Rheumatol. 1995;9(1):179–91.
Lupi O, et al. Tropical dermatology: bacterial tropical diseases. J Am Acad Dermatol. 2006a;54:559–78.
Paira SO, et al. The rheumatic manifestations of leprosy. Clin Rheumatol. 1991;10(3):274–6.
Rostom S, et al. Neurogenic osteoarthropathy in leprosy. Clin Rheumatol. 2007;26:2153–5.
Walker SL, et al. Leprosy. Clin Dermatol. 2007;25:165–72.
11.5 Toxoplasmosis
Toxoplasmosis is an opportunistic, protozoan infection of humans by Toxoplasma gondii. The name of the parasite is derived from the Greek word “toxon” meaning bow (the shape of the parasite) and “gondi,” which is a local name for a desert rodent in North America that hosts this parasite.
The life cycle of T. gondii occurs commonly between cats and mice. The cat (definite host) harbors the parasite in its intestinal mucosa, where sexual reproduction occurs to produce the oocysts. The oocysts are passed through the cat feces into the soil. The oocysts mature into the infective form within the soil, depending on the temperature and other conditions. In the mouse, the sporozoites invade the intestinal mucosa and are distributed via the blood and lymphatics through the body. The cycle is completed when the cat ingests a mouse infected with the parasite.
Humans get T. gondii incidentally, as intermediate hosts, by ingestion of the oocyst in uncooked meat (pork mutton) or food contaminated with household cat feces. The transplacental route of infection from mother to fetus is also a common method of toxoplasmosis infection. Ingestion of uncooked meat is an important route of transmission worldwide. Cooking meats at high temperatures (>66 °C) or freezing the meat for 1 day is sufficient to kill the parasite. Fast food that is improperly grilled or barbecued may still be infective, as the parasite is not killed.
Pregnant women should avoid cats, as toxoplasmosis infection is one of the known infections that can pass through the placenta from the mother to her fetus, along with other infections, such as rubella, cytomegalovirus, and herpes simplex virus (collectively called the TORCH complex). Infection of the mother before pregnancy rarely results in the birth of a congenitally infected child. Half the women who are infected with T. gondii during pregnancy do not transmit the parasite to their fetus. Fetal infection commonly occurs in the third trimester. The effect on the fetus is more significant when transmission occurs in the first trimester. Diagnosis is confirmed by serological detection of T. gondii antibodies in the amniotic fluid.
Multiple manifestations are noticed in patients infected with toxoplasmosis, depending on host immunity. In immunocompetent patients, fever, hepatosplenomegaly, enlarged lymph nodes, and headache may be seen. In contrast, immunocompromised patients show more extensive clinical symptoms that include lymphadenitis, fever, myocarditis, rash, meningitis, pneumonia, and encephalitis (50 % of cases). Moreover, fundoscopic examination of the retina often reveals yellowish, cotton-like patches within the globe.
In immunocompromised patients, toxoplasmosis can cause rheumatic diseases such as acute myositis that resembles polymyositis or dermatomyositis due to direct infection of the muscles or due to autoimmune reaction affecting the muscles. Other rheumatic manifestations include fever and rheumatic-like arthritis of the small joints, with fever that resembles adult Still’s disease and development of vasculitis and (rarely) Raynaud’s phenomenon.
Signs on US
In immunocompromised patients with toxoplasmosis, hepatosplenomegaly with retroperitoneal lymphadenopathy may be detected by ultrasound.
Signs on Brain CT
Toxoplasmosis commonly involves the basal ganglia, but other regions may be involved.
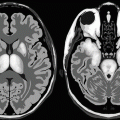
In congenital toxoplasmosis, brain CT characteristically shows hydrocephalus, parenchymal atrophy, and multiple scattered parenchymal calcifications often found around the lateral ventricles and the basal ganglia (Fig. 11.5.1). Hydrocephalus almost always arises due to aqueductal stenosis.

Fig. 11.5.1
Axial nonenhanced sequential CT images of a child born with congenital toxoplasmosis show brain parenchymal atrophy, moderate ventricular system dilatation (hydrocephalus), and characteristic calcification along the ventricular edges
Retinal calcifications may rarely be seen on CT in congenital toxoplasmosis due to retinochoroiditis (pathognomonic sign of ocular toxoplasmosis) (Fig. 11.5.2).

Fig. 11.5.2
Axial orbital CT illustration shows bilateral retinal calcification as a rare manifestation of toxoplasmosis (arrowheads)
In immunocompromised patients, solitary or multiple hypodense lesions surrounded by vasogenic edema, with ring contrast enhancement, are often detected (Fig. 11.5.3). Localization of the lesions in the basal ganglia is characteristic.

Fig. 11.5.3
Axial T1W postcontrast MRI shows toxoplasmosis lesion seen in an immunocompromised patient as a rounded lesion with vasogenic edema and ring enhancement (arrowhead)
Asymmetric target sign is a very characteristic sign of toxoplasmosis. There is an enhancing ring abscess that contains a similarly enhancing, eccentrically located nodule (Fig. 11.5.4). It is found in 30 % of cases.

Fig. 11.5.4
Axial postcontrast brain CT illustration demonstrates toxoplasmosis asymmetric target sign in the right centrum semiovale surrounded by vasogenic edema
A ringlike calcification may be seen in unenhanced images of treated toxoplasmosis lesions.
Toxoplasmosis is often difficult to differentiate from lymphoma. The subcortical location of toxoplasmosis compared with the subependymal location of lymphoma and the involvement of the corpus callosum in lymphoma that is not often seen in toxoplasmosis are helpful differentiating clues. Also, lymphoma is usually hyperdense on nonenhanced CT images, while toxoplasmosis becomes hyperdense on nonenhanced images only when the lesion is hemorrhagic or calcified.
Further Reading
Alappat JP, et al. A case of cerebral toxoplasmosis. Neurol India. 2000;48:185–6.
Diebler C, et al. Congenital toxoplasmosis. Clinical and neuroradiological evaluation of the cerebral lesions. Neuroradiology. 1985;27:125–30.
Dunn IJ, et al. Toxoplasmosis. Semin Roentgenol. 1998a;33(1):81–5.
Mombró M, et al. Congenital toxoplasmosis: assessment of risk to newborns in confirmed and uncertain maternal infection. Eur J Pediatr. 2003;162:703–6.
Navia BA, et al. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986;19:224–38.
Palm C, et al. Diagnosis of cerebral toxoplasmosis by detection of Toxoplasma gondii tachyzoites in cerebrospinal fluid. J Neurol. 2008;255:939–41.
Peng SL. Rheumatic manifestations of parasitic diseases. Semin Arthritis Rheum. 2002a;31:228–47.
Singh S. Mother-to-child transmission and diagnosis of Toxoplasma gondii infection during pregnancy. Indian J Microbiol. 2003;21(2):69–76.
Surendrababu NRS, et al. Globe calcification in congenital toxoplasmosis. Indian J Pediatr. 2006;73(6):527–8.
Yanagisawa S, et al. Ocular toxoplasmosis in Brazilians living in Japan. Ann Opthalmol. 2002;34(1):54–7.
11.6 Brucellosis (Malta Fever)
Brucellosis, also known as “Malta fever,” is a zoonotic disease caused by intracellular, gram-negative coccobacilli bacterium. Zoonosis is a term used to describe infections that are transmitted to humans from infected animals. The disease is named after the discoverer of the bacterium “David Bruce” in 1887. The name “Malta fever” is derived from the geographic endemic region where the fever is originally described.
Brucellosis is almost always transmitted to humans from infected animals. Different species of the bacteria are identified, and four species are responsible for most human infections: Brucella melitensis (found in sheep and goats), Brucella abortus (found in cattle), Brucella suis (found in swine), and Brucella canis (found in dogs). B. melitensis is the most common species infecting humans. The organism name is derived from Melita (honey), the Roman name for the Island of Malta.
Humans develop brucellosis after ingesting raw infected milk or dairy products such as cheese, yogurt, or ice cream prepared from unpasteurized milk. Camel milk is an important source of brucellosis infection in the Middle East and Mongolia.
For B. melitensis, a small infective dose of ten organisms is sufficient to initiate the disease. The incubation period is between 1 week and 10 months.
Brucellosis can infect any organ and may present with a variety of symptoms, depending on the infected organ. Patients typically present with a fever that can be acute (<2 months), subacute (2–12 months), or chronic (>1 year). The fever is typically normal during the early part of the day and rises during the night. Brucellosis is one of the common causes of pyrexia of unknown origin.
Other symptoms include influenza-like illness, sweating, malaise, myalgia, headaches, weight loss, lymphadenopathy, hepatosplenomegaly, and joint pain (arthralgia). Joint and back pain may be the first manifestations of brucellosis and is seen in up to 40 % of cases. Back pain arises either due to sacroiliitis or spondylitis. Peripheral arthritis is a common complaint and usually affects the knees, hips, and ankles.
Unilateral epididymo-orchitis is the most frequent complication affecting the genitourinary system.
The liver is commonly affected in brucellosis, and laboratory investigations often show liver enzyme abnormalities.
In 5–7 % of patients, the central nervous system is affected in the form of transient ischemic attacks, meningitis, encephalitis, and demyelinating diseases. Cranial nerves may be affected in neurobrucellosis, especially the optic, abducens, facial, and the cochlear branch of the vestibulocochlear nerve in the form of neuritis. Headache due to intracranial hypertension is a common symptom in neurobrucellosis. Diagnosis can be confirmed by identifying Brucella antibodies in the cerebrospinal fluid (CSF) or the serum. The organism is rarely isolated from the CSF.
The spine is commonly infected by brucellosis via hematogenous spread though the lumbar venous plexus. The lumbosacral region is the most frequently affected (60 %), followed by the thoracic region. Spondylodiscitis and vertebral osteomyelitis are common findings. Back pain and large joints arthralgia are described in up to 15 % of cases of chronic spinal brucellosis.
The skin is involved in 1–12 % of patients, mostly females, in the form of vasculitis or erythema nodosum. Up to 2 % of brucellosis deaths are attributed to Brucella endocarditis.
Brucellosis diagnosis is confirmed by demonstrating Brucella-specific antigens in the serum and blood culture (definite diagnosis) or by polymerase chain reaction performed on any clinical specimen.
Signs on Plain Radiographs
Spondylitis often begins in the superior vertebral end plates. The organisms are located in the anterior part of the end plate, initiating epiphysitis. Erosion and destruction of the anterior-superior part of the end plates with new bone formation is a characteristic sign of vertebral brucellosis (Pons’ sign) (Fig. 11.6.1).

Fig. 11.6.1
Lateral plain radiograph of the lower thoracic vertebrae in a patient with brucellosis shows spondylitis affecting the anterior-superior and the anterior-inferior vertebral end plates (arrowheads)
The healing process is marked by dense sclerosis, with the formation of anterior-superior end plate “parrot-peak” osteophytes.
Signs on US
Brucellosis epididymo-orchitis is seen as a focal, hypoechoic mass near the testes, with marginal flow signal on color flow Doppler sonography, reflecting hyperemia. The normal epididymis does not show high flow signal on color flow Doppler sonography.
Hydrocele and scrotal skin thickening may be found.
The resistance index may be reduced due to hyperemia, with low-resistance arterial flow pattern seen on pulsed Doppler sonography.
Signs on MRI
Signs of encephalitis or meningitis may be seen.
Enhancement of the cranial nerves is detected when neuritis is suspected clinically.
The main problem in diagnosing brucellosis of the spine is to differentiate it from tuberculosis (TB) of the spine. How can you differentiate between the two conditions?
Brucellosis commonly affects the lumbosacral vertebrae, while TB commonly affects the thoracic vertebrae.
The vertebral height is preserved in brucellosis, while it is severely damaged in TB.
The posterior elements and the epidural sac are usually spared in brucellosis, while they are affected in TB.
Further Reading
Bayram MM, et al. Scrotal gray-scale and color Doppler sonographic findings in genitourinary brucellosis. J Clin Ultrasound. 1997;25:443–7.
Bilen S, et al. Four different clinical manifestations of neurobrucellosis. Eur J Intern Med. 2008;19:e75–7.
Estevão MHL, et al. Neurobrucellosis in children. Eur J Pediatr. 1995;154:120–2.
Glasgow MMS. Brucellosis of the spine. Br J Surg. 1976;63:283–8.
Guney F, et al. First case report of neurobrucellosis associated with hydrocephalus. Clin Neurol Neurosurg. 2008;110:739–42.
Jochem T, et al. Neurobrucellosis with thalamic infarction: a case report. Neurol Sci. 2008;29:481–3.
Koc Z, et al. Gonadal brucellosis abscess: imaging and clinical findings in 3 cases and review of the literature. J Clin Ultrasound. 2007;35:395–400.
Mantur BG, et al. Review of clinical and laboratory features of human brucellosis. Indian J Med Microbiol. 2007;25(3):188–202.
Mays SA. Lysis at the anterior vertebral body margin: evidence for brucellar spondylitis? Int J Osteoarchaeol. 2007;17:107–18.
Metin A, et al. Cutaneous findings encountered in brucellosis and review of the literature. Int J Dermatol. 2001;40:434–8.
Tali ET, et al. MRI of brucella polyneuritis in a child. Neuroradiology. 1996;38:S190–2.
11.7 Neurocysticercosis
Cysticercosis is a parasitic disease caused by human infection with Taenia solium, the pork tapeworm.
The definitive host of T. solium is the pig. The larvae are ingested by humans in improperly prepared, infected pork meat. After ingestion, the larvae attach themselves to the intestinal mucosa and develop into adult tapeworms in 5–12 weeks. The tapeworm eggs contain active embryos (oncospheres), which are excreted in the stool. Pigs ingest the infected stool, and the oncospheres are liberated into pigs’ gastrointestinal tract, enter the mesenteric circulation, and develop into larvae in various tissues, completing the life cycle. Cysticercosis is endemic in parts of Asia, Thailand, India, Europe, and Latin America.
Cysticerci are found in various human tissues, but they have affinity for the central nervous system (neurocysticercosis). The clinical findings in neurocysticercosis are often nonspecific, and diagnosis is confirmed only by imaging and laboratory cerebrospinal fluid (CSF) studies. Patients commonly present with headaches, seizures (70 %), and neurological deficits. Arachnoiditis, infarction, and obstruction of the ventricular system by intraventricular lesions or reactive ependymitis may occur.
Neurocysticercosis can be found within the brain parenchyma, within the arachnoid space, the intraventricular space, and (very rarely) within the spinal cord (<1 % of cases).
Cisternal or subarachnoid cysticercosis is caused by two types of larval worms: Cysticercus cellulosae and Cysticercus racemosus. They are usually found in the basal cisterns, Sylvian fissures, or ventricles.
Signs on Plain Radiographs
When the larval cysts are killed by the inflammatory reaction within muscles and subcutaneous tissues, calcification of the dead cysts is seen as ovoid flecks of calcification resembling grains of rice (rice grain calcification). These calcifications are characteristic of cysticercosis and usually parallel the long axis of the muscle.
The CT and MRI findings in neurocysticercosis mainly depend on the stage of the disease; four stages are recognized:
Stage 1 (vesicular stage): in this stage (Fig. 11.7.1), the cysticerci are viable, with immune tolerance. There is a cystic lesion in the brain with little or no sign of acute inflammation, because the cyst is able to escape the host’s immune system surveillance. The cyst shows no contrast enhancement. A small eccentric nodule may be found within the cyst, which represents the parasite’s head or scolex (Fig. 11.7.2). This is referred to as hole–with–dot sign, and it is almost a pathognomonic sign of neurocysticercosis. Single or multiple cysts may be found anywhere within the brain. Patients are often asymptomatic in this stage.

Fig. 11.7.1
Axial brain CT illustration shows the four stages of neurocysticercosis: (1) vesicular stage, (2) colloidal stage, (3) granular stage, and (4) calcified stage

Fig. 11.7.2
Axial T1W postcontrast (a) and T2W (b) brain MR illustrations show different neurocysticercosis stages. In (a) and (b), the right cyst represents the vesicular stage, with eccentric scolex (arrowheads). The left cyst represents the colloidal stage, with rim contrast enhancement and edema around the cyst (arrows)
Stage 2 (colloidal stage): this stage develops after years, when the larvae start to die. The immune system starts an inflammatory response, and the fluid within the cyst becomes opaque. The cyst wall is thickened and shows contrast enhancement (Figs. 11.7.2 and 11.7.3). Edema around the lesions is demonstrated on T2W and FLAIR images.

Fig. 11.7.3
Axial T1W postcontrast (a) and T2W (b) brain MR images of colloidal stage neurocysticercosis (arrowheads)
Stage 3 (granular stage): in this stage, the colloid cyst is transformed into a nodular granuloma (Fig. 11.7.1). The lesion is nodular, with low T1/T2 signal intensities, surrounded by perifocal edema.
Stage 4 (calcified stage): in this stage, deposition of calcium occurs within the granuloma, and the lesion is calcified (Fig. 11.7.1). This stage is best demonstrated by CT.
Miliary neurocysticercosis: this uncommon form of neurocysticercosis is characterized by small (3–5 mm), bilateral symmetrical nodular cystic parenchymal lesions with marked edema (Fig. 11.7.4). This form is often seen in children and young adults.

Fig. 11.7.4
Axial T1W (a) and T2W (b) MR illustrations show the radiological appearance of miliary neurocysticercosis
Racemose neurocysticercosis is found in the subarachnoid space or the basal cisterns, with a similar signal and density to the CSF on MRI or CT, respectively. Racemose neurocysticercosis may manifest as a large lobulated (resembling bunch of grapes) cyst compressing the adjacent structures. It also frequently infiltrates the basal meninges, causing extensive meningitis and fibrosis. The cyst typically shows no scolex or contrast enhancement. The combination of a large lobulated cyst with no mural nodule inside it and enhanced basal meninges strongly suggests racemose neurocysticercosis, especially in endemic areas (Fig. 11.7.5).

Fig. 11.7.5
Coronal postcontrast T1W brain MR illustration demonstrates left lobulated cystic lesions within the Sylvian fissure (arrowhead), representing racemose neurocysticercosis with leptomeningitis ipsilaterally (arrow)
Intraventricular neurocysticercosis is seen as intraventricular round lesions with signs of hydrocephalus due to ventricular obstruction.
Intraspinal neurocysticercosis is seen on MRI as an intramedullary cystic mass with fluid signal with wall enhancement according to the stage. Serological testing of the CSF is helpful to establish the diagnosis.
Further Reading
Chang KH, et al. MRI of CNS parasitic diseases. JMRI. 1998;8:297–307.
Dumas JL, et al. Parenchymal neurocysticercosis: follow-up and staging by MRI. Neuroradiology. 1997;39:12–8.
Palacios E, et al. Computed tomography and magnetic resonance imaging of neurocysticercosis. Semin Roentgenol. 1997;32(4):325–34.
Roche CJ, et al. Selections from the buffet of food signs in radiology. RadioGraphics. 2002;22:1369–84.
Ruiz-García M, et al. Neurocysticercosis in children. Clinical experience in 122 patients. Child’s Nerv Syst. 1997;13:608–12.
Yeh SJ, et al. Neurocysticercosis presenting with epilepsia partialis continua: a clinicopathologic report and literature review. J Formos Med Assoc. 2008;107(7):576–81.
11.8 Ascariasis
Worms, also known as “helminthes,” are parasitic infections. Diagnosis is usually made by identifying the worm eggs in the stool.
Ascariasis is a parasitic disease that arises due to ingestion of food contaminated by the eggs of the roundworm (nematodes) Ascaris lumbricoides. Most patients are children between 1 and 15 years of age. Consuming uncooked vegetables and drinking polluted water from wells are important sources of ascariasis infection.
After ingestion of the eggs, the larvae hatch from the eggs before they reach the intestine, due to stimulation by gastric juices. The larvae penetrate the intestinal wall, enter the bloodstream, and travel via the portal venous or the lymphatic systems to the liver and then to the thoracic cavity. When they reach the lungs, the larvae grow and mature within the lung alveoli. When the worms are mature enough, they migrate from the lungs into the bronchi and from the trachea to the epiglottis, from where they are swallowed into the intestine for the second time. The matured larvae grow into adult worms in the intestine, especially the jejunum, and produce eggs that pass out in the feces. Up to 99 % of ascarids are found in the jejunum and ileum.
Most patients are asymptomatic, although severe ascariasis infection can cause abdominal cramps and malnutrition. The worms may also invade the gallbladder, appendix, liver, or bile duct. Ileocecal intestinal obstruction, ascending cholangitis, cholecystitis, appendicitis, and liver abscess are documented complications of ascariasis.
Respiratory symptoms in the form of fever, hemoptysis, cough, and pneumonia (ascariasis pneumonia) occur 5–26 days postinfection. The alveoli are filled with eosinophils and white blood cells attacking the larvae. Ascariasis is one of the most common causes of Loffler’s syndrome (fever, systemic eosinophilia, asthma, cough with sputum, and signs of alveolar infiltration on chest radiograph). The adult worm can produce a neurotoxin that can result in neurological manifestations (ascariasis encephalopathy).
Diagnosis is made by identifying the Ascaris eggs in the feces and pronounced eosinophilia on complete blood count.
Differential Diagnoses and Related Diseases
Visceral larva migrans (VLM) is a disease characterized by the invasion and residence of animal parasites in human tissues for a long time. The disease is often seen in children and often caused by Toxocara canis (from dogs) and Toxocara cati (from cats). Rarely, VLM can be caused by pig’s roundworm, Ascaris suum, which is closely related to human roundworm, Ascaris lumbricoides.
Signs on Chest Radiograph
Signs of patchy alveolar infiltration.
A pulmonary nodule can occur if the larvae form a granulomatous lesion when they die.
Signs on Ultrasound
In the gallbladder, the Ascaris worm is identified as a tubular structure with nondirectional movement causing a zigzag sign. The tubular structure has 3–4 parallel echogenic lines in longitudinal axis and a target sign in transverse axis.
When the gallbladder is full of worms, echogenic, intraluminal, and spaghetti-like structures are seen.
Signs on Barium Enteroclysis
The ascarides are seen as long, tubular filling defects within the intestinal lumen in the jejunum or the ileum (Fig. 11.8.1).

Fig. 11.8.1
Barium enteroclysis radiograph of a patient with ascariasis shows a long, tubular filling defect in the jejunum, with a double contrast sign representing Ascaris worm with barium ingestion (arrowhead)
The worm may ingest the barium, which will cause its gastrointestinal opacification, resulting in double contrast worm appearance around the barium (Fig. 11.8.1).
Signs on CT
On bowel oral contrast-enhanced CT, the worm is seen as a tubular filling defect within the bowel loops (Fig. 11.8.2). A thin enhanced line within the tubular defect can be seen representing contrast within the gastrointestinal tract of the worm due to contrast ingestion.

Fig. 11.8.2
Axial CT illustration demonstrates Ascaris worms within the intestinal bowel loops
In the gallbladder, the worms are seen as tubular, coiled soft-tissue structures within the gallbladder with no contrast enhancement. Speckles of curvilinear calcifications may be seen.
Further Reading
Hayashi K, et al. Hepatic imaging studies on patients with visceral larva migrans due to probable Ascaris suum infections. Abdom Imaging. 1999;24:465–9.
Kakihara D, et al. Liver lesions of visceral larva migrans due to Ascaris suum infection: CT findings. Abdom Imaging. 2004;29:598–602.
Maheshwari PR. Gall bladder ascariasis. Clin Radiol Extra. 2004;59:8–10.
Ochoa B. Surgical complications of ascariasis. World J Surg. 1991;15:222–7.
Reeder MM. The radiological and ultrasound evaluation of ascariasis of the gastrointestinal, biliary, and respiratory tracts. Semin Roentgenol. 1998;33(1):57–78.
Robbani I, et al. Worms in liver abscess: extensive hepatobiliary ascariasis. Dig Liver Dis. 2008;40(12):962. doi:10.1016/j.dld.2008.03.008.
Sherman SC, et al. The CT diagnosis of ascariasis. J Emerg Med. 2005;28(4):471–2.
Slesak G, et al. Obstructive biliary ascariasis with cholangitis and hepatic abscess in Laos: a case report with gall bladder ultrasound video. J Infect. 2007;54:e233–5.
11.9 Guinea Worm Disease (Dracunculiasis)
Dracunculiasis is an infection of the body by Dracunculus medinensis, a tissue-invasive round worm (nematode).
The name “medinensis” is derived from the frequency of human guinea worm infestation near Medina, a city in Saudi Arabia. It is a disease that is seen in the Middle East, Asia, and Africa.
The parasite enters the body through drinking water infected with the larvae, which penetrate the intestine and enter the bloodstream to lie deep within the subcutaneous tissues. The worm can grow under the skin up to 100 cm and usually exposes its uterus out of the host body through the skin to release its larvae into the water.
Patients infected with D. medinensis often present with allergic symptoms, nausea, and vomiting. Patients also present with skin blisters, sterile abscess, and (uncommonly) septic arthritis. The worm can be sensed under the skin within the abscess.
D. medinensis tends to migrate into the lower extremities, breast, and scrotum. Other sites in the body might be affected as well. It rarely affects the viscera.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree