(1)
Princess Elizabeth Hospital Le Vauquiedor St. Martin’s Guernsey, Channel Islands, UK
(2)
Brighton and Sussex Medical School, Brighton, England UK
Abstract
The term radial scar (strahlige Narbe) was introduced by Hamperl in 1975 to describe a lesion with a stellate configuration, which mammographically and pathologically mimics invasive carcinoma. This term is simple and avoids the use of other multiple synonyms, which do not always reflect the intrinsic pathological features. Some of the terms applied to the radial scar include the following: sclerosing papillary proliferation (Fenoglio and Lattes 1974), but papillary proliferation is not always present in these lesions; infiltrating epitheliosis (Azzopardi 1979), which has a malignant connotation; and indurative mastopathy (Rickert et al. 1981) and yet in the majority of cases the lesion is not always palpable. Fisher et al. (1979) applied the term nonencapsulated sclerosing lesion.
Learning Points
A radial scar mimics stellate cancer radiologically and pathologically.
There is no consensus as to whether a radial scar is a marker for risk of cancer or a precursor of breast cancer.
Needle core biopsies underestimate the presence of cancer in radial scars.
The diagnosis of radial scar on radiology and needle core biopsy should prompt an excisional biopsy.
Sclerosing adenosis can mimic cancer clinically, radiologically and pathologically.
Sclerosing adenosis is frequently identified as an incidental finding in specimens excised for benign or malignant conditions.
Immunocytochemistry markers are useful in sclerosing adenosis to demonstrate myoepithelial cells to confirm benignity.
Diagnosis of sclerosing adenosis in a needle core biopsy is usually accurate if there is no radiological–pathological discordance.
Apocrine adenosis is rare and its role as a precursor of malignancy has yet to be established.
Microgranular adenosis is unusual as a benign lesion because the glands lack myoepithelial cells.
Microglandular adenosis histologically mimics invasive carcinoma.
There is molecular evidence that microglandular adenosis may be linked to triple-negative breast cancer.
9.1 Radial Scar
The term radial scar (strahlige Narbe) was introduced by Hamperl in 1975 to describe a lesion with a stellate configuration, which mammographically and pathologically mimics invasive carcinoma. This term is simple and avoids the use of other multiple synonyms, which do not always reflect the intrinsic pathological features. Some of the terms applied to the radial scar include the following: sclerosing papillary proliferation (Fenoglio and Lattes 1974), but papillary proliferation is not always present in these lesions; infiltrating epitheliosis (Azzopardi 1979), which has a malignant connotation; and indurative mastopathy (Rickert et al. 1981) and yet in the majority of cases the lesion is not always palpable. Fisher et al. (1979) applied the term nonencapsulated sclerosing lesion.
Stellate lesions up to 9 or 10 mm can be classified as radial scars and, if larger, as complex sclerosing lesions (Anderson and Battersby 1985; Page and Anderson 1987). For simplicity, the term radial scar will be used in this book for benign stellate lesions. The incidence of radial scars in women undergoing screening mammography ranges from 0.04 % to 0.9 % (Wallis et al. 1993; Tabár and Dean 1985). The prevalence of radial scars in autopsy breast tissue is between 14 % and 28 % (Wellings and Alpers 1984; Nielsen et al. 1985). Incidental microscopic radial scars are commonly identified in breast tissue excised for benign or malignant disease. Although frequently screen-detected, palpable radial scars have also been reported (Wallis et al. 1993).
9.1.1 Pathogenesis of the Radial Scar
The evolution of the radial scar is ill understood, which may reflect the array of names applied to this lesion. Because of the frequent association of radial scars with fibrocystic change, some authorities believe they are a manifestation of fibrocystic change (Nielsen et al. 1985). Hamperl (1975) assumed that radial scars develop from obliterative processes involving the ducts. Fisher et al. (1983) postulated that radial scars arose from obliterative vascular processes. In an attempt to understand the pathogenesis of these lesions, Anderson and Battersby (1985) meticulously tabulated the histological features of 103 radial scars to assess common pathological features. They concluded that in the early stages, there was a secondary response to a central chronic inflammation with many spindle cells and minimal fibro-elastotic distortion of the parenchyma. In the late stage of the radial scar, there was considerable fibro-elastotic deposition associated with parenchymal distortion in a characteristic stellate configuration. As a follow-on to this study, in same periodical, in the same year, the same authors (Battersby and Anderson 1985) published results of an ultrastructural study on 38 radial scars. Twelve of these scars had associated carcinoma and the remaining 26 were benign. A third of the radial scars, which were regarded to be in the early stage of development, had abundant spindle cells. These cells had features of a myofibroblast on electron microscopy, which included endoplasmic reticulum and prominent myofilaments. The myofibroblasts had close association with collagen and elastic fibres. The ‘mature’ radial scars had few myofibroblasts remaining in the stroma. The stromal cells in the immature lesions were related to degenerating duct–lobular units, which frequently showed loss of basal lamina. The authors concluded that the obliteration of the central duct–lobular units appeared to be a prominent feature in the genesis of the radial scar. The presence of fibro-elastotic core is a common feature in radial scars which Wellings and Alpers (1984) used as the inclusion criterion when they carried out subgross analysis of radial scars. However, elastosis per se should not be applied to determine benignity, as elastosis occurs in both benign and malignant lesions (Azzopardi 1979).
Kulka et al. (1996) demonstrated morphological differences between benign and malignant stellate lesions when they examined 500–1,000 micron sections with infrared microscopy. The stellate extensions of complex sclerosing lesions (radial scar) consisted mostly of epithelial structures, whereas those of stellate cancers had a predominance of fibrous tissue.
9.1.2 Radiological Features of the Radial Scar
Radial scars are small and usually impalpable stellate (spiculate) lesions, which are often identified in breast tissue excised for cancer or other benign proliferations (Andersen and Gram 1984; Nielsen et al. 1985, 1987). Spiculated lesions can be a result of benign or malignant processes and these include sclerosing adenosis, postsurgical scar, radial scar, post-traumatic oil cysts and infiltrating ductal carcinoma (Franquet et al. 1993). The radial scar is a prototype stellate lesion, which mammographically can be indistinguishable from a carcinoma (Fig. 9.1). Mammographic features that have been found to be common to radial scars include radiolucent central core, elongated radiating spicules and infrequent calcification (Fig. 9.2). Fenoglio and Lattes (1974) attributed the central radiolucency to the presence of a hypertrophic fibroelastic core, which is surrounded by radiating (stellate) proliferations of the duct system.
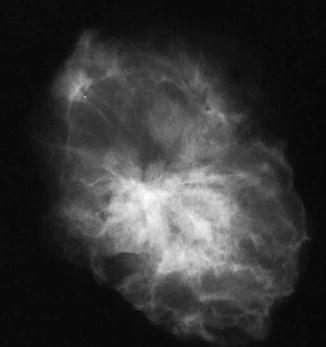
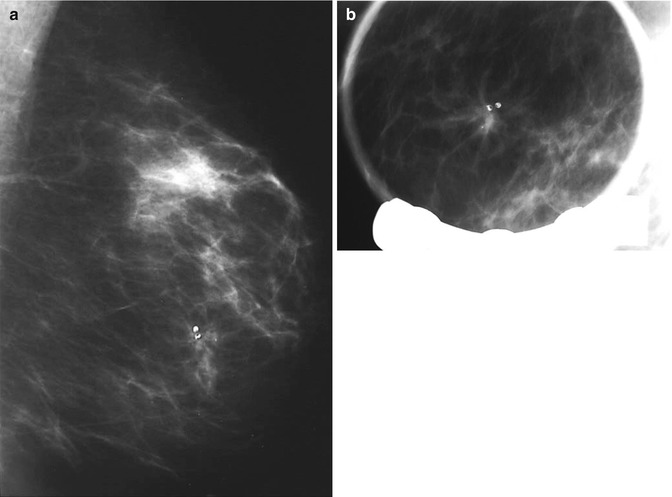

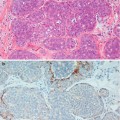
Fig. 9.1
The specimen X-ray shows an ill-defined stellate lesion, which was present as an architectural distortion in the left breast during mammographic screening. The lesion is better defined in the specimen X-ray than in vivo

Fig. 9.2
(a) Screen-detected area of architectural distortion with focal calcification. (b) The magnification view shows enhanced stellate configuration
In the early days of screening mammography, the absence of calcification in stellate lesions was thought to discriminate radial scars from carcinomas. However, in a retrospective review of 225 consecutive stellate lesions, Mitnick et al. (1989) found that the radiological features used to distinguish benign from malignant stellate lesions were unreliable but indicated that the presence of calcification could be useful. They reported 14 out of 73 (19 %) carcinomas with radiological features of a radial scar. The presence of microcalcification in the 11 patients with carcinoma assisted the authors to distinguish carcinoma from radial scar. Four of the nine biopsy-proven radial scars had a dense central region simulating the appearance of a scirrhous carcinoma. From these findings the authors concluded that stellate lesions with radiolucent cores associated with calcification were suggestive of carcinoma. However, in a separate study, Adler et al. (1990) reported that the presence of calcification or radiolucent core was not a useful criterion in differentiating carcinomas from radial scars. The presence of calcification in radial scars has also been reported in several publications (Nielsen et al. 1987; Orel et al. 1992; Franquet et al. 1993). Although the calcification is not always apparent in the in vivo mammogram, it may be highlighted in the specimen radiographs (Adler et al. 1990). King et al. (2000) reviewed the mammographic and pathological features of 45 patients who had undergone localisation excision for radial scar. Only ten patients had mammographic and histological evidence of radial scar. Twenty-nine patients had only mammographic and six had only histological features of a radial scar. Carcinoma was identified in 18 patients with mammographic features of radial scars.
The sonographic features of radial scars are not diagnostic either. The lesions produce ill-defined images with poor echogenicity and shadowing. The radiating spicules make it difficult to differentiate a radial scar from carcinoma (Cosgrove and Svensson 2001). However, Cohen and Sferlazza (2000) suggested that sonography may be useful in assessing subtle mammographically detected radial scars, especially when they are present in only one view and cannot be specifically localised. Because of the lack of pathognomonic features on radiological analysis, stellate lesions should be biopsied and managed appropriately. MRI did not differentiate benign radial scars from those harbouring malignancy (Cha et al. 2007), but was found useful in a separate study by Perfetto et al. (2009).
9.1.3 Pathological Features of Radial Scar
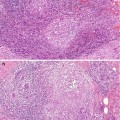
A microscopic or evolving radial scars can be identified in tissue excided for other benign of malignant lesion (Fig. 9.3). The hallmark of the radial scar is the presence of central fibro-elastotic core which may contain blood vessels. However, not all radial scars have the elastotic component and central fibrous tissue may contain tubular structures to mimic tubular carcinoma (Fig. 9.4). Calcification may be present in the central fibrous core (Fig. 9.5). The fibrous tissue radiates outward to give rise to the characteristic star-like appearance (Fig. 9.5). The proliferation at the periphery of the radial scar is variable and can include cysts, florid UDH, ADH, papillomas, fibrocystic with apocrine metaplasia, columnar cell lesions and early invasive carcinoma (Kennedy et al. 2003). The radial scar in Fig. 9.5 shows cysts with associated florid UDH at the periphery. The main differential diagnosis of a radial scar is a stellate cancer. Some of the stellate cancers contain a fibro-elastotic core which makes some authorities believe that these cancers develop in a radial scar (Fig. 9.6). The fibro-elastotic core in Fig. 9.6 is better developed than that in the radial scars above, which provides compelling evidence for advocates of the radial scar as a precancerous lesion.
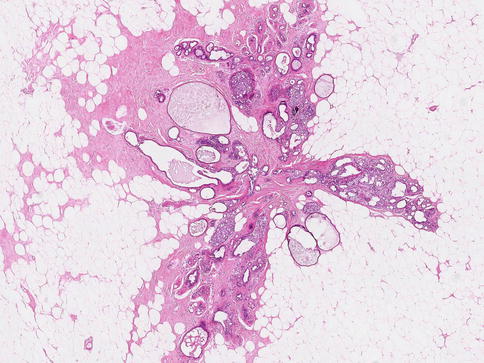
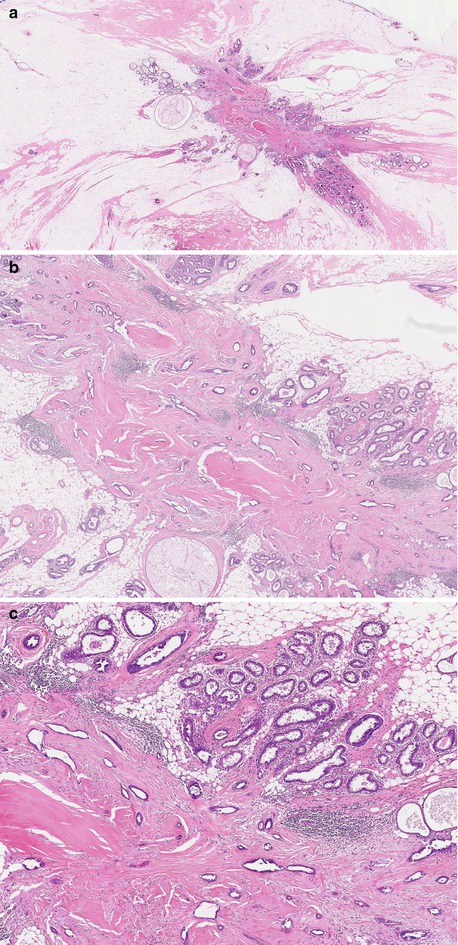
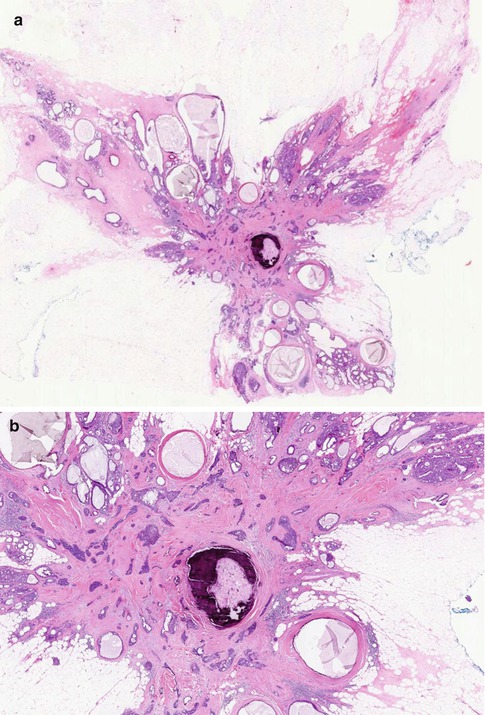
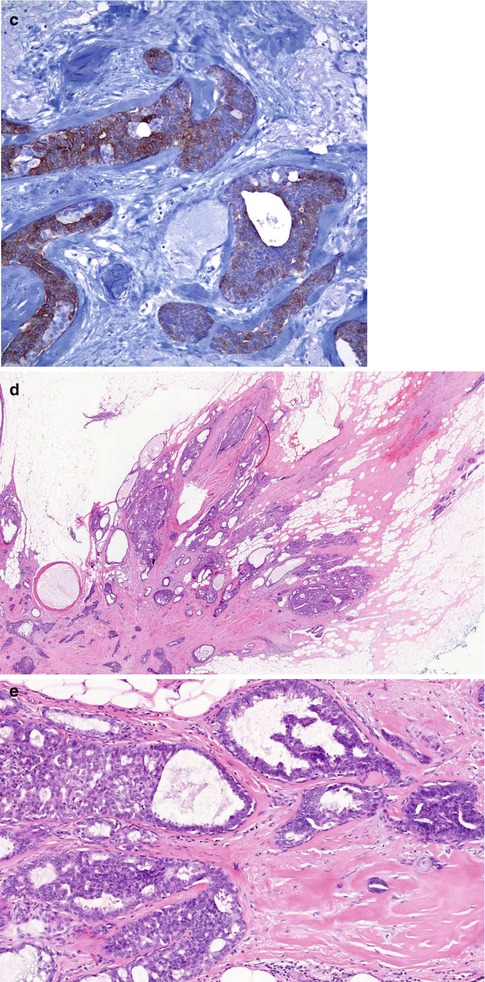
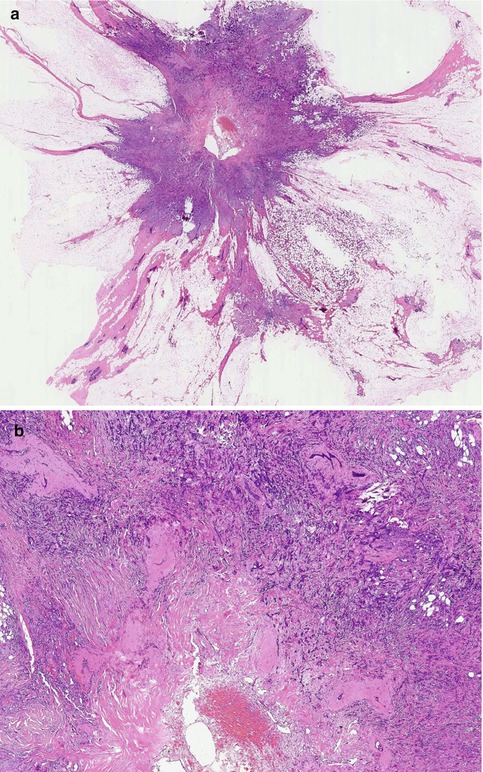

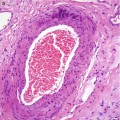
Fig. 9.3
Microscopic evolving radial scar as an incidental finding in a mastectomy specimen for breast cancer. Even at this stage, the typical radiating morphology is apparent

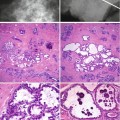
Fig. 9.4
(a) Whole mount section of a radial scar with central fibrosis, but no elastotic features. (b) High power of the central core to illustrate the lack of elastosis; tubular structures mimicking tubular carcinoma are evident in the central fibrotic stroma and (c) Columnar cell change at the periphery of the fibrotic core (same lesion in Fig. 9.1)


Fig. 9.5
(a) Whole mount section of large radial scar, 25 mm in diameter, with central fibro-elastotic core and focal calcification. (b) In the core there are islands of entrapped epithelial cells. (c) Entrapped epithelial cells are positive with CK5/6 which confirms benignity. (d) The fibrous radiations contain cysts and prominent usual ductal hyperplasia (UDH). (e) High magnification highlights UDH in a fibro-elastotic stroma (same lesion in Fig. 9.2)

Fig. 9.6
(a) Stellate cancer which can mimic a radial scar radiologically. (b) High magnification illustrates the fibro-elastotic core, which is better developed here than that in the radial scars above
9.1.4 Management of Patients with Radial on a Needle Core Biopsy
If a radial scar is detected mammographically, the general recommendation is to excise it as cancer cannot be excluded. The UK NHS Breast Screening Programme classifies a radial scar in a needle core biopsy as a B3 category lesion (NHSBSP Publication No 50 2001), but how frequent is the upgrade of benign radial scar on biopsy to a high-grade lesion such as DCIS or invasive cancer in the excision biopsy? In a retrospective review of 4,458 consecutive image-guided needle core biopsies performed at the University of Udine in Italy, between 2000 and 2008, Linda et al. (2010) identified 79 lesions diagnosed as radial scars (1.8 %); these 62 radial scars in 62 women were analysed in the study as they had pathology of the excision specimen. Of the 62 lesions, five (8 %) were reported as malignant at surgical excision (3 DCIS; 1 IDC; 1 ILC; none were tubular); 40 (65 %) were graded as high-risk lesions (33 radial scars; 6 ADH; 1 FEA) and 17 (27 %) benign lesions (18 fibrocystic change; 7 sclerosing adenosis; 2 UDH). In this study, percutaneous biopsies underestimated malignancy in 5/62 (8 %). Neither mammographic nor ultrasonographic features could predict the lesions which would yield cancer in the excision specimens. The authors pointed out that underestimation of malignancy in radial scars occurs due to sampling error but also from the diagnostic error in the difficulty in differentiating a radial scar from tubular carcinoma. In this study Linda and co-authors (2010) reviewed 11 publications from 1996 to 2008 and the underestimation of cancer in biopsies reported as radial scars was 0–40 %. In a separate study, López-Medina et al. (2006) and co-authors identified eight cancers (18 %) in 43 excision specimens of patients with a diagnosis of radial scar on needle core biopsy. Morgan and colleagues (2012) reported a similar prevalence to Linda et al. (2010) of six cancers (9 %) identified in 67 patients with a diagnosis of radial scar on needle core biopsy. Based on these findings the overall consensus is that all patients with percutaneous diagnosis of radial scar should undergo surgical excision regardless of mammographic or sonographic features.
9.1.5 Molecular Features of the Radial Scar
Jacobs and colleagues (2002) performed in situ hybridisation on several stromal elements in nine radial scars, 15 cases of normal tissue and four invasive carcinomas. Compared with normal tissue, the radial scars showed focal increased blood vessels using immunocytochemistry to factor VIII-related antigen. The in situ hybridisation showed focally increased expression of mRNA for collagen type 1, total fibronectin and other stromal and vascular elements. The pattern of mRNA expression in the radial scars was similar to that seen in the four invasive carcinomas. The authors concluded that the similarities between radial scars and invasive breast cancers with regard to mRNA expression for several stromal and vascular factors suggest similar disturbances in stromal–epithelial interactions in both lesions. In a separate study, Iqbal and colleagues (2002) demonstrated allelic imbalance and LOH, on p16 and 8p which are genetic alterations usually present in breast cancer. These results suggest clonal proliferation and possibly a precursor of breast cancer. In the same study, Iqbal and co-authors also demonstrated LOH and allelic imbalances in different types of proliferation associated with radial scar which included hyperplasia of usual type, sclerosing adenosis and apocrine metaplasia suggesting that the radial scar consists of a mixture of multiple proliferations, potentially clonal which were independent from lesions outside the radial scar. The authors surmised the radial scar was an independent risk factor for breast cancer but also provided a cumulative risk in association with other benign breast disease. They also postulated that this would explain the increased risk of malignancy associated with larger radial scars (Sloane and Mayers 1993; Jacobs et al. 1999). Iqbal and colleagues could not confirm whether the radial scar was a marker for risk of malignancy or a premalignant lesion and recommended further studies with a wider range of genetic markers.
9.1.6 Is the Radial Scar a Premalignant Lesion?
The frequent presence of tubular structures within the fibro-elastotic core of the radial scar, which closely resembles tubular carcinoma, has led to the belief that radial scars represent an early stage of tubular carcinoma (Fisher et al. 1983; Linell and Rank 1989). Carcinoma arising in association with radial scars is well documented, but there is no consensus as to whether or not these lesions are premalignant or just markers of subsequent risk of malignancy. Linell and Rank (1989) believed that the corona of the radial scar consists of contracted lobules and ducts around a sclerotic/elastotic centre. These contracted lobules and ductules were demonstrated to be part and parcel of the terminal duct–lobular units, which are the origin of cancer (Wellings et al. 1975). From their well-illustrated monogram, Linell and Rank (1989) demonstrated that some tubular carcinomas arose in radial scars. In addition to the elastotic centre, they emphasised the fact that both tubular carcinomas and radial scars are stellate shaped and are rarely more than 10 mm in diameter. This view that radial scars are precursors of tubular carcinoma is not widely accepted. In a subgross analysis of radial scars, Wellings and Alpers (1984) identified only a single case of ductal carcinoma in situ when they examined 83 radial scars. These authors concluded that a radial scar is at least a ‘marker’ for risk of cancer development.
In a separate study, Nielsen and colleagues (1987) examined breast tissue from 84 consecutive autopsies of women with clinical diagnoses of invasive breast cancer. Radial scars were found in contralateral breasts in 35 patients (42 %). Four women had radial scars in the ipsilateral breast with cancer. One woman had invasive cancer with morphological features compatible with, but not diagnostic of transition from a radial scar. Six radial scars had associated ductal or lobular carcinoma in situ and two had atypical hyperplasia. The frequency of radial scar was significantly higher in women with fibrocystic disease (55 %) compared to women without (24 %). Although this study did not imply that radial scars were potentially premalignant, the authors noted that radial scars containing high-risk epithelial proliferations such as atypical hyperplasia and carcinoma in situ which were associated with an increased risk of subsequent breast cancer.
Sloane and Mayers (1993) reviewed 126 radial scars and complex sclerosing lesions to determine factors associated with malignancy and atypical hyperplasia. They reported a clear relationship between the size of the lesion and the presence of atypical hyperplasia. Atypical hyperplasia and carcinoma were uncommon in lesions less than 6–7 mm but more frequent in larger lesions. Complex sclerosing lesions with carcinoma and atypical hyperplasia were also common in women older than 50 years and infrequent below the age of 40. The types of carcinomas associated with the radial scars included small and large cell DCIS, LCIS, tubular and ductal carcinoma. As most radial scars or other stellate lesions are screen-detected, this study emphasises the significance of excising these lesions to exclude malignancy, especially in older women.
9.1.7 The Radial Scar as a Risk Factor of Subsequent Cancer
In an attempt to ascertain the cancer risk associated with a radial scar, Jacobs et al. (1999) carried out a case–control study on 1,396 women who had had biopsies for benign breast disease. The women were followed up for 12 years and 99 (7.1 %) had a diagnosis of radial scar. Most of the radial scars were incidental microscopic findings with an overall median size of 4.0 mm. Single radial scars were present in 60.6 % of the women. Thirty-two per cent (32/99) of the women with radial scars developed breast cancer compared to 17 % (223/1,297) of the control group without radial scars. Women with radial scars tended to be older (>45 years) and most likely to be postmenopausal than those without radial scars. These findings partly concurred with the results of Sloane and Mayers (1993). There was no relationship between the presence of radial scar, age at menarche, parity, age at birth of first child or body mass index.
Jacobs et al. (1999) also assessed the benign disease associated with the radial scars and classified this into nonproliferative and proliferative disease without atypia and atypical hyperplasia. The RR of developing subsequent breast cancer in women with proliferative disease without radial scar was 1.5 and this doubled to 3.0 in women with radical scar. Women with atypical hyperplasia but no radical scar had an RR of 3.8; this increased to 5.8 when the women had a radial scar. These relative risks were adjusted for age, year of biopsy of benign breast lesion and follow-up interval. Adjustment for other breast cancer risk factors such as age at menarche, family history of breast cancer, body mass index, menopausal status, parity and age at birth of first child did not significantly alter the RR. Women with nonproliferative benign breast disease with a single radial scar had an RR of 2.5 compared with similar women with multiple radial scars with an RR of 4.3. The presence of atypical hyperplasia increased the RR from 3.5 in women with single radial scars to 8.4 when multiple radial scars were present. When the size of the radial scars was taken into account, lesions larger than 4 mm had a higher RR than smaller lesions. Women with proliferative disease without atypia associated with radial scars of 4.0 mm had an RR of 3.5, and this increased to 8.8 when atypical hyperplasia was present. There was not much difference between proliferative disease without atypia (RR = 2.4) and atypical hyperplasia RR = 2.0 when radial scars less than 4.0 mm were present. From this study, Jacobs and colleagues postulated that the increased risk associated with radial scars could be related to disturbances between the interaction of the epithelial and stromal elements. In addition to highlighting the risk associated with radial scars, this study is also important to remind pathologists assessing benign breast disease to include the size and the number of radial scars. Jacob’s study was on symptomatic patients with incidental radial scars. As radial scars tend to affect older women (>50 years) who are in the screening age group, separate studies are required to assess the risk of screen-detected radial scars.
Sanders et al. (2006) recorded lower RR than Jacobs et al. (1999). The authors followed up 880 women with a diagnosis of radial scar enrolled in the Nashville Breast Cohort for 20.4 years. Sixty-two (7 %) women developed invasive breast cancer compared to 5.5 % controls. The RR of developing invasive breast cancer was 1.82 (95 % CI, 1.2–2.7) at 10 years. When the analysis was restricted to women over 49 years of age, the RR increased to 2.14 (95 % CI, 0.6–2.8). The RR declined with increasing years of follow-up. Approximately 92 % of the women with radial scars had proliferative disease, the RR of radial scar with associated atypical hyperplasia was 6.72 (95 % CI, 2.5–18). These authors attributed the increased risk of subsequent cancer to the presence of proliferative disease rather than the radial scar per se. Similarly, Andersen and Gram (1984) had previously followed up 32 women with radial scars without atypical epithelial proliferation for a mean of 19.5 years (range 15–24 years) and only one patient developed cancer.
9.2 Sclerosing Adenosis
9.2.1 Pathogenesis and Clinical Features
Foote and Stewart (1945) initially described sclerosing adenosis as a lesion that presented as a palpable symptomatic tumour or as a microscopic incidental finding. Sclerosing adenosis arises as a result of proliferation of the terminal duct–lobular units in a disordered manner caused by an increase in acinar and myoepithelial cells and stromal elements (Jensen et al. 1989). In 1970, Tanaka and Oota (1970) carried out a detailed stereomicroscopic study of thick sections to ascertain the pathogenesis of mastopathic breast disease. From the stereomicroscopic images, the authors described sclerosing adenosis as proliferating parallel arrays of ductules, which sprouted abruptly from large flattened ducts to create ‘bundles of noodles’ forming knots or whorls. They noted that lobular formation was rarely seen in these lesions, which is in contradiction to the widely held view that sclerosing adenosis is a lobulo-centric process (Wellings et al. 1975). Sclerosing adenosis can be identified as a component of other proliferative lesions such as radial scars or fibroadenomas. Sclerosing adenosis has the ability to mimic cancer clinically and radiologically. Patients can present clinically with a palpable mass or detected radiologically through the screening process.
9.2.2 Radiological Features of Sclerosing Adenosis
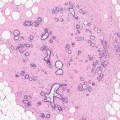
In the screening age group, sclerosing adenosis can be detected as the main pathological lesion or identified incidentally in the background of other lesions, benign or malignant. In Lanyi’s series (1986) of 52 patients who underwent biopsies for mammographically detected clustered microcalcification, sclerosing adenosis was detected in 15 patients (28.8 %). Nineteen out of 38 patients with diffuse milk of calcification cysts had a diagnosis of sclerosing adenosis on biopsy which he termed microcystic adenosis (Fig. 9.9c).
Microcalcification, radial structures or a combination of the two are the main mammographic features of sclerosing adenosis. The calcification in sclerosing adenosis is polymorphous (Fig. 9.7), and Lanyi believes this develops as the lobular cysts containing milk of calcium and psammoma bodies become ‘distorted’ by the proliferating myoepithelial cells and fibrous tissue (Fig. 9.9). However, the calcification in sclerosing adenosis is not always apparent radiologically but can be detected on histology. In some cases of sclerosing adenosis excised on the basis of clustered microcalcification, the calcification was present in the adjacent cysts, not in the compressed acini of sclerosing adenosis (Lanyi 1986). Although mammographically the pattern of calcification in sclerosing adenosis is variable, round- or rosette-shaped clusters are suggestive of sclerosing adenosis. Extensive diffuse microcalcification is rare (Lanyi 1986). Sometimes the calcification can be quite atypical and simulates malignant calcification (MacErlean and Nathan 1972; Lanyi 1986). When a mass lesion is present, sclerosing adenosis may appear multilobulated, focal, diffuse, nodular or spiculated (Cyrlak et al. 1999).
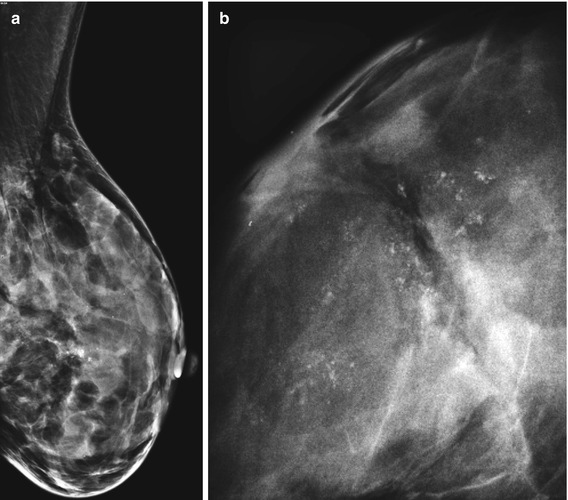
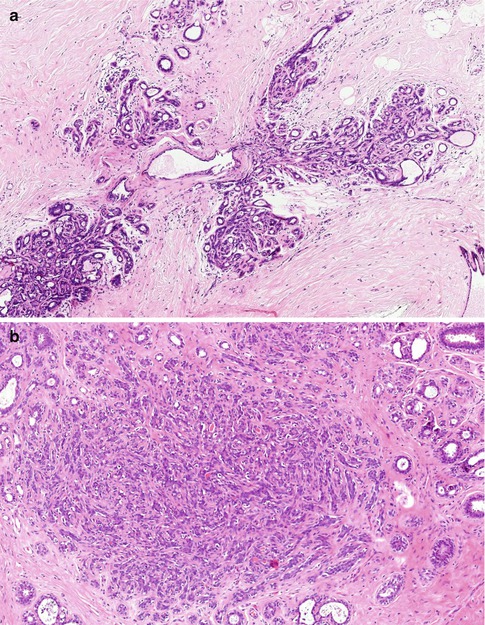

Fig. 9.7
(a) A large area of coarse calcification due to sclerosing adenosis which was graded as R4. (b) High magnification at the time of stereotactic biopsy. The biopsy was reported as calcified sclerosing adenosis (B2) but the abnormality was excised due to radiological–pathological discordance

Fig. 9.8
(a) Incidental microscopic sclerosing adenosis in a specimen excised for other abnormality. Note the expansion of the TDLUs with a hyalinised stroma. (b) Is another microscopic sclerosing adenosis with a fibroblastic stroma
DiPiro and colleagues (2000) reviewed the mammographic and sonographic features of 12 patients (age range 26–61) with histologically proven nodular adenosis. Four patients presented symptomatically and in eight women the lesions were detected radiologically. Mammography was performed in ten patients; the lesions had a lobular circumscribed pattern in seven women and indistinct partly obscured or completely obscured masses in three women. One lesion contained mammographically heterogeneous calcifications. Ultrasonography was performed in seven patients and five revealed oval masses, one was lobular and another was irregular. All lesions were hypoechoic and six were circumscribed. No lesion revealed posterior acoustic shadowing of sound and five masses had posterior acoustic enhancement. Because the mammographic and sonographic features of nodular adenosis were not specific, the authors recommended needle core or excisional biopsy. Nielsen and Nielsen (1986) did not find any radiological pathognomonic features when they reviewed 18 cases of histologically verified adenosis tumours.
9.2.3 Pathological Features of Sclerosing Adenosis
Sclerosing adenosis is a common finding in breast specimens excised for other lesions. When sclerosing adenosis presents with a tumour-forming lesion, the cut surface has a white fibrous appearance with ill-defined margins, but generally there are no specific macroscopic features.
Histologically there is proliferation of the TDLUs characterised by an increase in the number of acini that may produce a mass (florid adenosis) or in the extreme (adenosis tumour) or become surrounded by stromal sclerosis (sclerosis adenosis) (Nielsen 1987




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree