Renal enlargement
Decreased or increased parenchymal echogenicity
Minimal dilatation of renal pelvis
Loss of normal corticomedullary differentiation
Focal, poorly defined mass with variable echogenicity
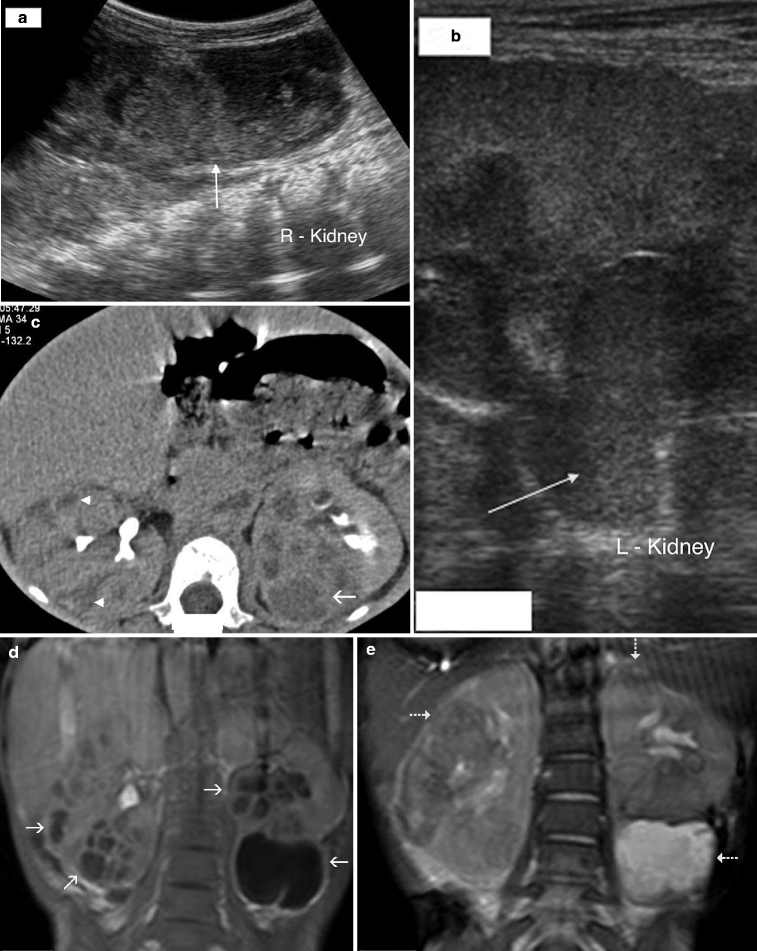
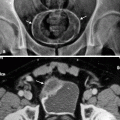
Fig. 2.1
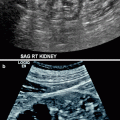
Complicated acute bacterial pyelonephritis (ABP) in a 3-year-old girl with immunodeficiency. Ultrasound scans of right (a) and left (b) kidneys reveal bilateral diffuse enlargement with loss of the corticomedullary differentiation (arrow), multiple scattered areas of decreased echogenicity, and dilated renal pelvis (arrow) representing diffuse spread of the inflammatory process, features consistent with complicated acute pyelonephritis, and pyonephrosis. (c) Contrast-enhanced CT scan demonstrates bilateral diffuse renal involvement with scattered nonenhancing foci representing microabscesses (arrowheads) and a large cystic fluid collection in the left kidney (arrow) suggestive of a renal abscess. The maximum intensity projection image from excretory magnetic resonance urography (MRU) (d) and the maximum intensity projection image from T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence (e) reveal the true pathology that is consistent with bilateral multifocal renal abscesses associated with complicated ABP (dashed arrows)
Focal pyelonephritis or lobar nephronia is a localized infection without major suppuration. It usually appears as a poorly defined mass with focal loss of the regional corticomedullary differentiation on ultrasound. The abnormal focus can be relatively anechoic with low-level echoes or can be more echogenic than the kidney.
Renal ultrasound is also useful in the diagnosis of complications secondary to acute pyelonephritis such as abscess, pyonephrosis, and perirenal fluid collections (Figs. 2.1 and 2.2a, b).
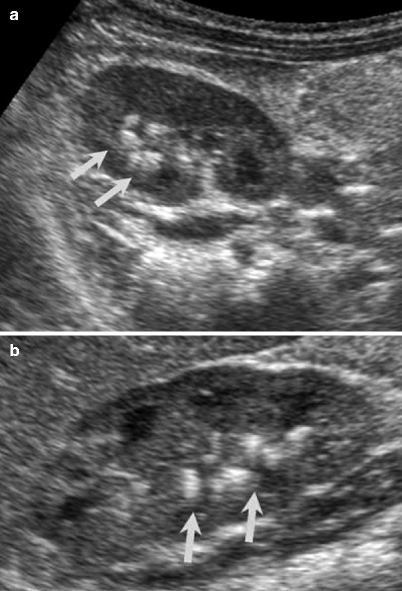

Fig. 2.2
Acute bacterial pyelonephritis. Transverse (a) and longitudinal (b) ultrasound scans of a 13 months old boy performed after an episode of UTI reveal echogenic caliceal debris associated with ABP (arrows) (Courtesy of Alparslan Ünsal, MD, Aydın, Turkey)
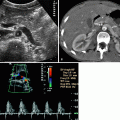
Combining gray-scale ultrasound and power Doppler ultrasound can provide a comprehensive evaluation of the renal parenchyma in acute pyelonephritis with impaired renal function.
Power Doppler ultrasound is superior to color-flow Doppler in defining extent of areas of hypoperfusion because most pyelonephritic lesions are ischemic.
Computed Tomography
Computed tomography (CT) is a sensitive study in evaluation of the renal parenchyma in acute renal infection.
Inflammatory edema and microabscess may be demonstrated in the abnormal renal parenchyma.
Rounded or irregular parenchymal abnormalities appear as a patchy low-density areas on nonenhanced CT scans.
After administration of radiographic contrast, these regions are seen as areas of decreased enhancement (Fig. 2.1c).
Magnetic Resonance Imaging
Parenchymal alterations in complicated renal infections can be demonstrated on MRI.
Abscess formations appear as hypointense on T1-weighted and hyperintense on T2-weighted images (Fig. 2.1d, e).
Nuclear Scintigraphy
In children, radionuclide imaging is strongly recommended both at early and late stages of the acute pyelonephritis because of the potential of significant functional deficits within areas of inflammation and suppuration.
Radionuclide imaging with Tc-99m dimercaptosuccinic acid (DMSA) is considered the most sensitive modality for the imaging of renal cortex in children.
It may show areas of peripheral decreased uptake related to acute pyelonephritis or scar formation.
Differential Diagnosis
The differential diagnosis of acute pyelonephritis is given in Table 2.2. Focal pyelonephritis causing a focal area of edema should be differentiated from various mass lesions, including a renal abscess, which usually appears more complex.
Table 2.2
Differential diagnosis of acute pyelonephritis in children
Ureteric obstruction
Contusion
Tubular obstruction
Hypotension
Scarring
Autosomal recessive polycystic kidney disease
Renal abscess
Pearls and Pitfalls
It is difficult to exclude the diagnosis of acute pyelonephritis with imaging studies because IVP, CT, and ultrasound findings can be normal in up to 75 % of the cases.
On ultrasound, abnormal echogenicity areas can occasionally be misinterpreted as mass lesions.
Although renal infection can be localized by DMSA, it occasionally cannot be definitely distinguished from a sterile inflammation by using any kind of radiotracer.
Acute Bacterial Renal Infection in Adults
General Information
The incidence of acute bacterial pyelonephritis (ABP) is high, with an increasing rate of hospitalization.
In the United States, approximately 250,000 cases of ABP with more than 100,000 hospitalizations occur each year.
Imaging
Intravenous Pyelography
The main role of IVP in the imaging of infection involves screening for obstruction, although this is now better demonstrated by other modalities.
Only 25 % of cases of ABP will have positive IVP findings, including enlargement of the affected kidney, delayed contrast excretion, and compression of the collecting system.
Ultrasound
Sonographically, variable echotexture changes of the renal parenchyma have been described for ABP.
They are in the form of hypoechoic lesions or to a lesser degree, hyperechoic foci.
The former are attributed to edema, whereas the latter may be due to hemorrhage.
Ultrasound can also be used to detect and monitor the progress of focal inflammatory masses.
Thus, ultrasound is widely used either as a complement to the IVP or as a screening modality, which may or may not be followed by CT scan, depending on the findings.
Computed Tomography
CT is considered the imaging modality of choice in the evaluation of patients with ABP.
It is superior to both ultrasound and IVP in detecting morphologic and functional abnormalities, defining the extent of disease, and detecting the formation of renal subcapsular or perinephric abscess or hematoma (Fig. 2.3a–c).
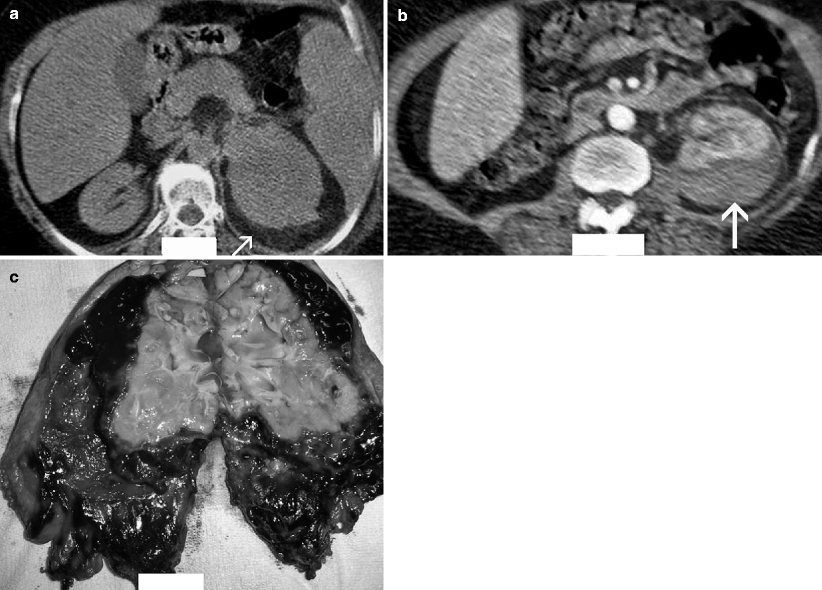
Fig. 2.3
Hemorrhagic acute pyelonephritis in a 45-year-old woman presented with left flank pain. (a) Nonenhanced CT scan demonstrates a low-attenuation collection in the left perirenal space (arrow). (b) Contrast-enhanced CT scan reveals a perirenal fluid collection with mixed attenuation (arrow) and thickening of Gerota’s fascia. Preoperative diagnosis was perirenal abscess. (c) Left nephrectomy revealed an acute pyelonephritis with perirenal hematoma
In hemorrhagic bacterial pyelonephritis, nonenhanced CT may demonstrate, on occasion, wedge-shaped or rounded areas with increased attenuation due to parenchymal bleeding.
Nonenhanced CT is an excellent study for identifying calculi, gas formation, hemorrhage, parenchymal calcification, urinary obstruction, renal enlargement, and inflammatory masses.
Typical findings of ABP after intravenous contrast administration include ill-defined, wedge-shaped lesions of decreased attenuation radiating from the papilla in the medulla to the cortical surface.
These wedge-shaped lesions probably represent areas of poorly or nonfunctioning parenchyma due to vasospasm, tubular obstruction, and/or interstitial edema.
The nonspecific appearance of a striated nephrogram in the excretory phase on CT comprises linear bands of alternating hyper- and hypoattenuation parallel to the axes of tubules and collecting ducts, corresponding to obstructed tubules with intervening normal tubules, which is associated with stasis of contrast material in dilated ducts due to edematous renal parenchyma (Table 2.3).
Table 2.3
Causes of striated nephrogram on CT
Acute pyelonephritis
Acute urinary obstruction
Renal vein thrombosis
Renal contusion
Hypotension
Medullary sponge kidney
Autosomal recessive polycystic kidney disease
Tubular obstruction (myoglobinuria)
Pathology
Acute bacterial pyelonephritis results from an ascending infection that originates in the lower urinary tract.
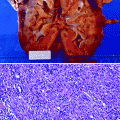
Grossly, the collecting system is thickened, with yellow-white abscesses located throughout the renal parenchyma. An abscess is composed of central necrosis, surrounded by a zone of preserved neutrophils, all of which is surrounded by dilated vessels and fibroblasts. Fibroblastic proliferations serve to organize and confine processes and cause adjacent structures to contract. Cortical abscesses may result in hematogenous spread, further seeding the kidney with microorganisms (Fig. 2.4).
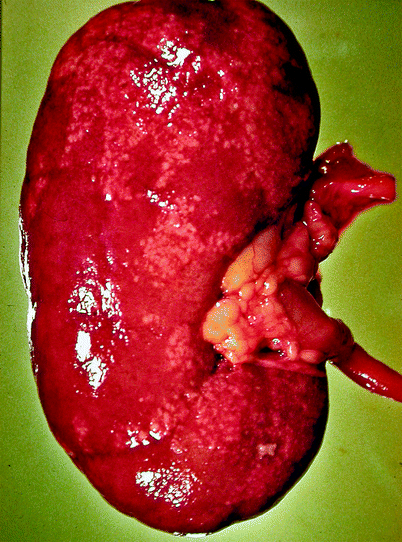
Fig. 2.4
Acute pyelonephritis. Patient died of sepsis after inadvertent ligation of one ureter. The kidney is swollen and pale. Microabscesses are visible on the cortical surface (From MacLennan GT, Cheng L. Atlas of Genitourinary Pathology. New York: Springer; 2011. Reprinted with permission)
Histologically, the predominant finding is neutrophils; neutrophils are present in the interstitium and in the renal tubules (eventually forming white blood cell casts) (Fig. 2.5). In early infection, neutrophils and bacteria may be seen in the collecting ducts.
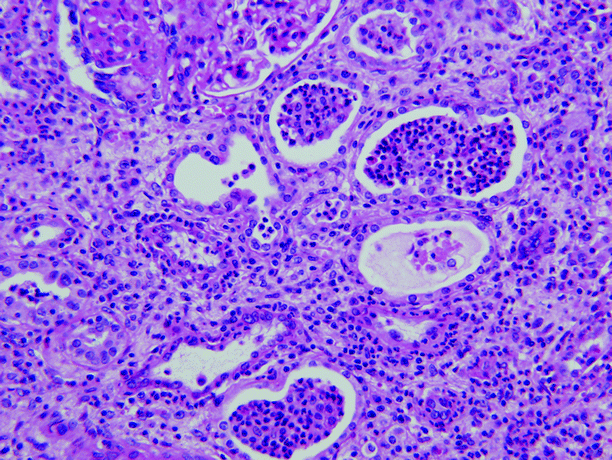
Fig. 2.5
Acute pyelonephritis. Abundant neutrophils are present in the interstitium and within renal tubules
Differential Diagnosis
On CT, differential diagnosis for focal areas of hypoattenuation includes renal infarction, tumors (renal cell carcinoma), and scarring.
The hypoattenuating areas associated with infarcts and tumors persist following antibiotic treatment, in contrast to those caused by ABP.
Furthermore, scarring rarely occurs in adult patients.
Pearls and Pitfalls
On ultrasound, the combination of a hypoechoic area and a focal inflammatory bulge to the perinephric space may be misinterpreted as a neoplasm.
On CT, streak artifacts during the excretory phase may give a false impression of abnormal nephrogram.
The appearance of a “striated nephrogram” is not pathognomonic for ABP and can be seen in kidneys following renal trauma, in medullary sponge kidney and in autosomal recessive polycystic kidney disease (Table 2.3).
Chronic Pyelonephritis
General Information
Chronic pyelonephritis is a chronic interstitial nephritis that may arise secondary to a host of etiologic causes, including recurrent infection, autoimmune diseases, calculous disease, and chronic obstruction.
Imaging
The imaging findings of chronic pyelonephritis are summarized in Table 2.4.
Table 2.4
Imaging findings in chronic pyelonephritis
Renal scarring |
Renal atrophy |
Cortical thinning |
Hypertrophy of residual normal tissue |
Caliceal clubbing |
Dilatation of the calyceal system |
Renal asymmetry |
Intravenous Pyelography
Renal size is usually decreased, unless chronic obstruction is present, in which case the renal size may be increased.
Renal contour blurring.
Cortical thinning.
Focal nonfunctional area in nephrogram phase.
Ultrasound
Focal or diffuse cortical thinning
Renal scar formation
Distortion of renal contours
Computed Tomography
The kidney is usually shrunken and has an irregular outer margin (Fig. 2.6).
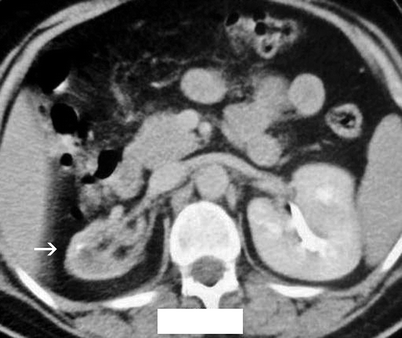
Fig. 2.6
Forty-year-old woman with recurrent pyelonephritis. Contrast-enhanced CT scan reveals severe parenchymal scarring of and decreased contrast excretion from the right kidney (arrow). The left kidney is normal
Renal scar formation presents as defect in the margin of the kidney between two calyces.
Decreased contrast excretion on enhanced CT.
Fibrotic distortion of the collecting system.
Pathology
Chronic pyelonephritis is a general term implying chronic interstitial renal infection due to long-standing obstruction or vesicoureteric reflux, resulting in loss of renal parenchyma, with scarring. Chronic pyelonephritis accounts for 5–15 % of cases of end-stage renal failure. Most patients are asymptomatic until hypertension or uremia develops.
The gross findings in chronic pyelonephritis are variable. When the condition is obstruction related, the kidney may be large due to dilatation of the collecting system, and the cortical surface is distorted by irregular scars (Fig. 2.7). Beneath the scars, the renal cortex is thin, and the papillae are scalloped or obliterated, causing the affected calyces to appear dilated.
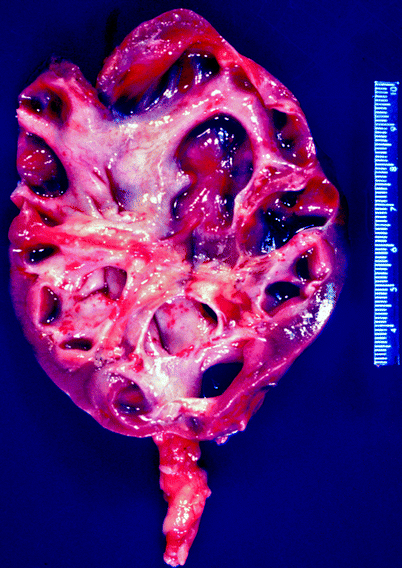
Fig. 2.7
Chronic obstructive pyelonephritis. The renal damage in a setting of obstructed renal drainage results from pressure-related atrophy and chronic infection. This patient had long-standing distal ureteral obstruction. The renal pelvis and calyces are dilated, with flattening or obliteration of papillae and global thinning of renal cortex
Microscopically, chronic pyelonephritis consists of interstitial fibrosis, tubular atrophy, and sclerosis of glomeruli, often patchy in distribution (Fig. 2.8). The interstitial inflammation is mixed but is composed mainly of lymphocytes. Tubules usually contain eosinophilic material, a feature known as “thyroidization.” Arteries and arterioles commonly show fibrosis, mural hyalinization, and occlusion.
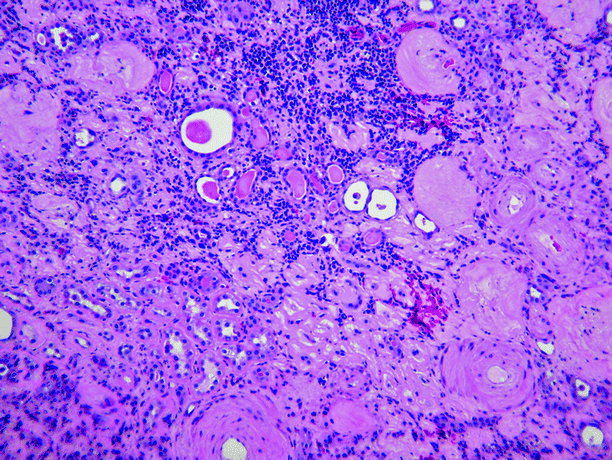
Fig. 2.8
Chronic obstructive pyelonephritis. The glomeruli are sclerotic, the interstitium shows fibrosis and is infiltrated by lymphocytes, the tubules are atrophic and contain eosinophilic casts (thyroidization), and the blood vessels show intimal sclerosis and mural thickening
Differential Diagnosis
Several conditions involving the loss of renal parenchymal tissue such as infarction or papillary necrosis and fetal lobulation should be considered in the differential diagnosis of renal scarring.
Pearls and Pitfalls
On ultrasound, the limited depiction of calyces may preclude the distinction of a renal scarring in cases with chronic pyelonephritis.
In chronic pyelonephritis, tubules usually contain eosinophilic material, a feature known as “thyroidization.”
Vesicoureteral Reflux and Reflux Nephropathy
General Information
Vesicoureteral reflux (VUR) is described as the existence of retrograde urine flow from the bladder to the ureter and the upper urinary tract.
This condition usually develops secondary to the primary abnormality of the vesicoureteral junction and may cause focal or diffuse acute and/or chronic pyelonephritis.
The prevalence of VUR in asymptomatic children is less than 0.5 %, but VUR is seen in up to 50 % of children with UTIs. Renal damage, also termed “reflux nephropathy,” develops in only 10 % of the children with symptomatic UTI.
Imaging
Intravenous Pyelography
IVP can demonstrate cortical contour deformity associated with a clubbed calix.
In the past, IVP was the preferred diagnostic method in the demonstration of renal scarring and parenchymal function but was replaced by ultrasound and DMSA renal scintigraphy because of its low sensitivity in identifying these abnormalities, the nephrotoxic potential of contrast materials used, and the necessity for exposure to radiation.
Voiding Cystoureterography
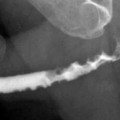
The gold standard method for the evaluation of VUR is the conventional voiding cystoureterography (VCUG) (Fig. 2.9a).
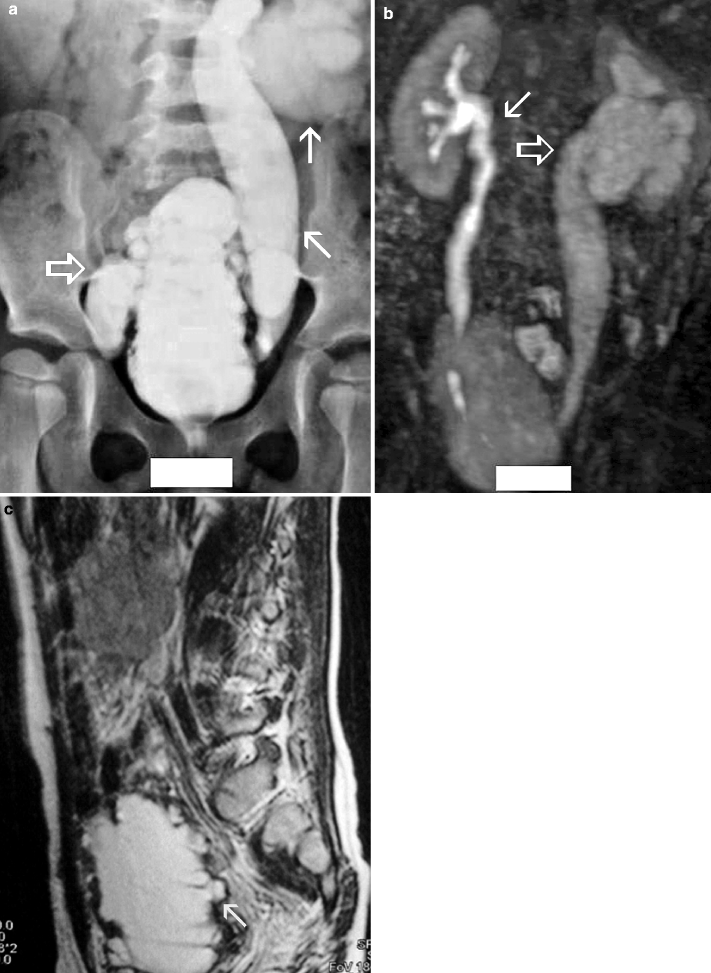
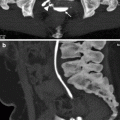
Fig. 2.9
VUR and reflux nephropathy in 5-year-old boy with recurrent bladder infection. (a) Retrograde voiding cystourethrogram reveals marked dilatation of the left ureter and left renal pelvicaliceal system (arrows) representing reflux to the left kidney and trabeculations and multiple diverticula in the bladder (open arrow). (b) Coronal contrast-enhanced 3D T1-weighted gradient echo MRI demonstrates minimal dilatation of the right collecting system (arrow) and severe hydroureteronephrosis on the left collecting system (open arrow). (c) On sagittal contrast-enhanced T1-weighted gradient echo MRI, bladder diverticula (arrow) and diffuse bladder wall thickening secondary to cystitis are visualized excellently
The international system of radiologic grading of vesicoureteral reflux has assigned five grades of reflux on VCUG:
Grade 0: Indicates no VUR
Grade 1: VUR that does not reach the renal pelvis
Grade 2: VUR extending to the renal pelvis without dilatation
Grade 3: VUR extending to the kidney with mild ureteral dilatation and mild to moderate pelvicalyceal dilatation
Grade 4: VUR extending to the kidney with moderate ureteral dilatation and complete obliteration of the sharp angles of all fornices
Grade 5: VUR extending to the kidney with a tortuous ureter and moderate dilatation of the renal pelvis to extreme dilatation of the entire upper urinary tract
The sensitivity of VCUG depends on the severity of the VUR. It has been demonstrated that VCUG has 100 % sensitivity in the diagnosis of grade 4 and grade 5 VUR.
Ultrasound
Ultrasound may be helpful for the detection of parenchymal damage and dilatation of the collecting system.
Features of VUR that may be evident by ultrasound include diminished renal length and dilatation of the ureter, the renal pelvis, and the renal calyces.
Ureteral dilatation was found to be the most helpful diagnostic feature in detection of the higher grades of VUR by ultrasound.
Recently, it has been advocated that echo contrast-enhanced cystosonography may be useful in diagnosing, grading, and following up on patients with VUR.
Magnetic Resonance Imaging
Multiplanar cross-sectional T1- and T2-weighted magnetic resonance imaging (MRI) combined with unenhanced 3D magnetic resonance urography (MRU) is potentially useful for morphological evaluation of the urinary system, without the hazards related to the use of intravenous iodinated contrast agents.
In addition to morphological evaluation, contrast-enhanced MRU provides functional information about renal perfusion, concentration, and excretion of contrast agent (Fig. 2.9b, c).
Gadolinium-enhanced T1-weighted excretory MRU may demonstrate the parenchymal scars and perfusion defects associated with reflux nephropathy.
Probably, the greatest advantage of the use of this T1-weighted gadolinium-enhanced 3D-MRU technique is the visualization of the urinary tract of patients with impaired renal function.
Unenhanced T2-weighted MRU technique allows excellent visualization of the urinary tract and is useful in patients with dilated collecting systems. However, a nondilated urinary tract is either invisible or incompletely visualized with this technique, whereas excellent visualization is achieved with contrast-enhanced T1-weighted excretory MRU.
Nuclear Scintigraphy
Direct radionuclide cystography with a 99mTc-labeled agent (sulfur colloid, diethylenetriamine penta-acetate [DTPA], or pertechnetate) is a well-accepted alternative to fluoroscopic VCUG for screening asymptomatic children, for follow-up examination of children with vesicoureteral reflux (VUR), for postoperative evaluation after ureteral reimplantation, and for excluding VUR.
99mTc-labeled DTPA scintigraphy is more sensitive than VCUG in detection of VUR.
DMSA renal scintigraphy has been found to be more sensitive than IVP in the evaluation of focal renal scars.
Pathology
When chronic pyelonephritis is primarily reflux related, the affected kidney is usually small. Reflux nephropathy usually results from vesicoureteral reflux (a congenital disorder) combined with intrarenal reflux, thus allowing infection access to the renal parenchyma.
The kidneys are small and contracted, with broad depressed sometimes saddle-shaped scars in the capsule (Fig. 2.10). There is loss of renal pyramids with dilated and thickened calyces, and the renal cortex overlying these areas is thinned. Renal parenchyma between scarred areas may be normal or hypertrophic.
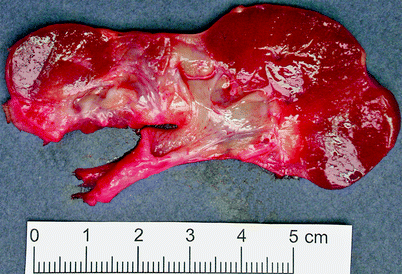
Fig. 2.10
Reflux nephropathy. Some portions of renal cortex are relatively preserved; in the scarred areas, there is virtually no residual cortex. This is an example of an Ask-Upmark kidney, part of a clinical scenario including chronic urinary tract infections associated with vesicoureteric reflux, hypertension, and a small kidney with saddle-shaped scars
When these findings are present in a hypertensive patient known to have had long-standing VUR, the renal lesion is sometimes described as “Ask-Upmark kidney” (Fig. 2.11).
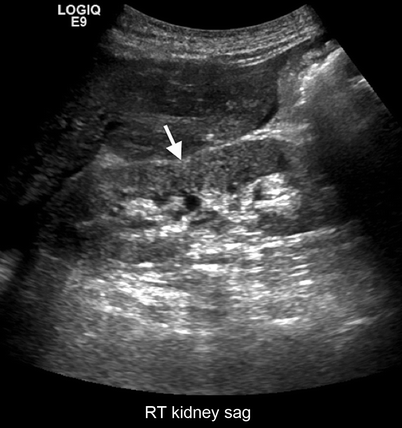
Fig. 2.11
Ask-Upmark kidney. Gray-scale ultrasound demonstrates saddle shape of the kidney (arrow) secondary to scar formation from VUR
The predominant histologic finding is an interstitial lymphoid infiltrate with extensive renal tubular atrophy. Residual tubules contain eosinophilic casts (thyroidization) (Fig. 2.12). Additional findings include glomerular and vascular sclerosis.
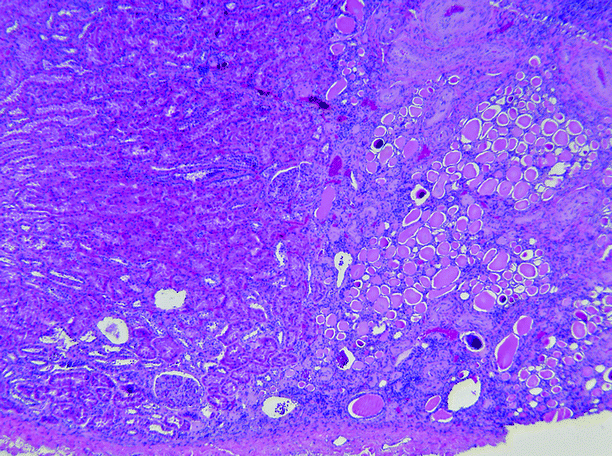
Fig. 2.12
Reflux nephropathy. In this Ask-Upmark kidney, the renal tissue on the left is essentially normal, whereas on the right, in the area of scarring, glomeruli are absent, and the interstitium is fibrotic and infiltrated by lymphocytes. The tubules are atrophic and contain eosinophilic casts (thyroidization), and the blood vessels show intimal sclerosis
Differential Diagnosis
On VCUG, scarring from reflux nephropathy should be differentiated from scarring due to other etiologies such as parenchymal infarction.
The deformed appearance of the calyx underlying the scar in reflux nephropathy may be a clue for the differential diagnosis.
Pearls and Pitfalls
Fetal lobation and other normal variants of renal development may be mistaken for renal scarring on ultrasound.
With VCUG, misinterpretation may be possible, for example, the soft tissue of the bowel wall may infrequently simulate contrast in a ureter, or overlying stool and intestinal gas may obscure the kidney, precluding the diagnosis of lesser degrees of VUR.
HIV-Associated Nephropathy
General Information
The kidneys are affected in HIV-infected patients by a wide spectrum of entities including HIV-associated nephropathy, atypical infections, malignancy, and drug-related renal disease.
The nephropathy may be the first manifestation of HIV infection and often precedes opportunistic infections.
It is the leading cause of renal failure in HIV-positive patients.