Inherited and Neurodegenerative Abnormalities
Andrea Poretti
Lee Finkelstone
Gul Moonis
QUESTIONS
1 A 40-year-old immunocompetent otherwise healthy male presents with recurrent migraine and progressive cognitive decline. An MRI was performed; key images are provided below.
|
1a What is the most likely diagnosis?
A. Alzheimer disease
B. Creutzfeldt-Jakob disease
C. CADASIL
D. Progressive multifocal leukoencephalopathy
View Answer
1a Answer C. CADASIL. In a young patient with FLAIR abnormality most notably within the anterior temporal lobes and frontal subcortical white matter, CADASIL is the most likely diagnosis.
1b What is the mechanism for developing CADASIL?
A. Etiology is unknown and felt to be idiopathic.
B. Inherited mutation on chromosome 17
C. Sporadic mutation in the p53 gene
D. Inherited mutation in the NOTCH3 gene
View Answer
1b Answer D. Inherited mutation in the NOTCH3 gene. CADASIL is a hereditary disease with mutation in the NOTCH3 gene. NF1 is a disease with an inherited mutation on chromosome 17, and p53 is an important tumor suppressor gene.
|
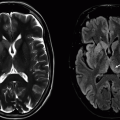
Imaging Findings: Multifocal hyperintense FLAIR signal abnormality is primarily within the bilateral anterior frontal lobe and temporal subcortical white matter (Figures A and C). In a young patient, this distribution of disease favors CADASIL as the diagnosis.
Discussion: CADASIL is short for cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. CADASIL is a hereditary disease secondary to a mutation in NOTCH3 gene on chromosome 19 leading to strokes in young to middle age adults who are otherwise healthy without stroke risk factors. CADASIL typically presents at age 30 to 50 with TIA/stroke-like symptoms, and many patients also present with migraine headaches with aura. The disease is progressive with cognitive decline and early death. Lacunar infarct burden has been shown to have an important impact on cognitive function and disability.
On imaging, CADASIL classically demonstrates relatively bilaterally symmetric FLAIR hyperintense signal abnormality within the paramedian superior frontal lobe subcortical white matter, anterior temporal lobes, and external capsules. The anterior temporal lobe involvement has been shown to be both highly specific and sensitive for aiding in the diagnosis of CADASIL. Cerebral microbleeds on gradient echo imaging are often also seen.
Differential Diagnosis: The main differential considerations for CADASIL include other vascular disorders or demyelinating disease. In atherosclerotic microvascular change, patients tend to be older and with white matter changes in the corona radiate/centrum semiovale as well as deep gray matter structures with sparing of the anterior temporal lobes. Demyelinating disease white matter hyperintensities are classically periventricular, oriented perpendicular to the ventricular system, and with corpus callosum involvement as well as the brainstem, cerebellum, and spinal cord.
References: Markus HS, et al. Diagnostic strategies in CADASIL. Neurology 2002;59(8):1134-1138.
Stojanov D, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015;15(1):1-8.
Viswanathan A, et al. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology 2007;69(2):172-179.
2 A 55-year-old male with a palatal tremor presented for further evaluation to a neurologist. Key images from an MRI are provided below.
|
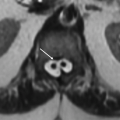
2a The location of pathology in the medulla (above) is bordered on either side by these structures.
A. Retroolivary sulcus and preolivary medullary sulcus
B. Fascicles of hypoglossal nerve and vagal, glossopharyngeal, and accessory nerves
C. Inferior cerebellar peduncle and lateral fossa of the medulla
D. Pyramid and fascicles of hypoglossal nerve
View Answer
2a Answer A. Retroolivary sulcus and preolivary medullary sulcus. The pathology is located in inferior olivary nucleus, which is bordered by preolivary sulcus (12th nerve emerges from this sulcus) and retro-olivary sulcus (9, 10, and 11 nerve fibers are in this sulcus).
2b What is the most likely diagnosis?
A. Neoplasm
B. Demyelination
C. Hypertrophic olivary degeneration
D. Medullary infarction
View Answer
2b Answer C. Hypertrophic olivary degeneration. Location is the key for diagnosis. Search for a causative lesion in Guillain-Mollaret triangle shows signal abnormality in contralateral dentate nucleus (probably representing gliosis).
|
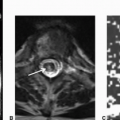
Imaging Findings: Axial FLAIR images demonstrate enlargement and increased signal within the left olive in A, with gliosis in the right dentate nucleus in B.
Discussion: Hypertrophic olivary degeneration (HOD) is caused by lesion in the triangle of Guillain and Mollaret. Patients can be asymptomatic or present with palatal tremor.
The triangle of Guillain and Mollaret (dentato-rubro-olivary pathway) contains a set of connecting tracts and is defined by the contralateral dentate nucleus (DN), ipsilateral inferior olivary nucleus (ION), and ipsilateral red nucleus (RN). The red nucleus is functionally not a part of the circuit. The efferent fibers from the DN ascend in the superior cerebellar peduncle (SCP) and after superior cerebellar peduncular decussation descend to the contralateral ION via central tegmental tract. Efferents from the ION go across the midline, travel via the inferior cerebellar peduncle cerebellar cortex, and then to the dentate nucleus, completing the triangle.
|
HOD is associated with lesions in afferent fibers to ION (first two limbs of the triangle). So, HOD is seen with lesions in contralateral dentate nucleus and ipsilateral brain stem. Transsynaptic degeneration is the proposed mechanism for HOD. Lesions of the olivodentate fibers can cause cerebellar atrophy. During the first month or so following the ictus, generally, there won’t be any imaging changes in ION. Then, three distinct stages of HOD have been described on MRI.
Acute stage with increased T2 signal without hypertrophy, first 6 months after ictus
Both increased signal and hypertrophy of ION, 6 months to 3 to 4 years after ictus
Disappearance of hypertrophy with some persistence of increased signal
Reference: Goyal M, Versnick E, Tuite P, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. Am J Neuroradiol 2000;21(6):1073-1077.
3 An 8-year-old boy presents with hearing loss, decreasing school performance, attention problems, and progressive gait abnormalities.
|
What is the most likely diagnosis?
A. Metachromatic leukodystrophy
B. X-linked adrenoleukodystrophy
C. Canavan disease
D. MELAS
E. Alexander disease
View Answer
3 Answer B. X-linked adrenoleukodystrophy.
|
Imaging Findings: Axial T2-weighted MR image shows symmetric T2 hyperintense signal in the medial geniculate nuclei. Axial T2-weighted MR image shows symmetric T2 hyperintense signal in the parietooccipital white matter with sparing of the U-fibers. Axial contrast-enhanced T1-weighted MR image shows a rim of enhancement surrounding the parietooccipital white matter.
Discussion: X-linked adrenal leukodystrophy (ALD) is caused by a pathogenic variant within the ABCD1 gene on chromosome Xq28 mutation. This gene codes for a peroxisomal membrane protein that plays a key role in the transport of very long chain fatty acids into the peroxisome, where they are normally metabolized. Hence, the analysis of very long chain fatty acids in plasma can be used as a diagnostic biomarker for ALD.
There are various clinical phenotypes among patients with X-linked ALD including the childhood cerebral form, adrenomyeloneuropathy (an adult form of ALD that presents with slowly progressive spastic paraparesis, impaired vibratory sense in the lower extremity, and bladder or bowel dysfunction and is characterized by a primary spinal cord involvement), and ALD with Addison disease only. The childhood cerebral variant of ALD presents at the age of 4 to 8 years with behavior problems, difficulties in school, and rapid regression of auditory discrimination, spatial orientation, speech, and writing.
The most common neuroimaging pattern in the childhood cerebral form of ALD consists of symmetric and predominantly parietooccipital white matter abnormalities that typically start in the splenium of the corpus callosum. The U-fibers and the cerebellum are relatively spared in the early onset of the disease, whereas the geniculate bodies, the lateral inferior part of the thalamus, and the posterior limb of the internal capsule may be affected early. In about 20% of the patients, the primary involvement involves the frontal white matter. After injection of contrast, a rim of enhancement may be noted surrounding the abnormal white matter. Enhancement seems to be associated with clinical worsening of the disease.
Differential Diagnosis: For the classic pattern of childhood cerebral ALD, there is almost no differential diagnosis. Alexander disease is a differential diagnosis of the ALD form with frontal predominance. Associated clinical features are key for differentiation.
References: Engelen M, et al. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis 2012;7:51.
Loes DJ, et al. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology 2003;61(3):369-374.
Moser HW, et al. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol 2007;3(3):140-151.
4 A 17-year-old teenager presents with a 4-year history of worsening psychiatric and cognitive issues and acute-onset hallucinations.
|
What is the most likely diagnosis?
A. Metachromatic leukodystrophy
B. X-linked adrenoleukodystrophy
C. Canavan disease
D. MELAS
E. Alexander disease
View Answer
4 Answer A. Metachromatic leukodystrophy.
|
Imaging Findings: Axial T2-weighted MR images show a T2 hyperintense signal in the deep and periventricular white matter with sparing of the U-fibers. In addition, horizontal stripes of relatively spared white matter are noted.
Discussion: Metachromatic leukodystrophy is an autosomal recessive lysosomal disease caused by deficiency of arylsulfatase A activity leading to the accumulation of galactosylsulfatide in the white matter of the central and peripheral nervous system.
Late infantile-onset metachromatic leukodystrophy represents the most common phenotype (60% to 70%). Affected children typically present with progressive gait problems at about 14 to 16 months of age. The disease is progressive, and affected patients may lose developmental milestones and develop dysarthria, drooling, dysphagia, rigidity, and decerebrate or decorticate postures. Later, manifestations are typically characterized by behavioral abnormalities, cognitive deficits, and dementia.
MR images are nonspecific and may show areas of T2/FLAIR hyperintense signal in the deep and periventricular cerebral white matter, whereas the subcortical white matter (U-fibers) is spared until late in the course of the disease. Stripes of affected and unaffected myelin (called a “tigroid” pattern) may be seen and represent relatively spared myelin and lipid-containing glial cells in the perivascular spaces.
Differential Diagnosis: Periventricular predominance of white matter signal changes may be seen in several (hereditary and acquired) white matter diseases including Krabbe disease, Sjögren-Larsson disease, leukoencephalopathy with brainstem and spinal cord involvement and high lactate (LBSL), periventricular leukomalacia, and HIV leukoencephalopathy. The “tigroid pattern” may be seen in Krabbe disease and GM1 gangliosidoses. Hematopoietic stem cell transplant, if performed early in metachromatic leukodystrophy, can not only stabilize but even improve cerebral white matter abnormalities.
References: Eichler F, et al. Metachromatic leukodystrophy: a scoring system for brain MR imaging observations. AJNR Am J Neuroradiol 2009;30(10):1893-1897.
Eichler FS, et al. Metachromatic leukodystrophy: an assessment of disease burden. J Child Neurol 2016;31(13):1457-1463.
Groeschel S, et al. Cerebral gray and white matter changes and clinical course in metachromatic leukodystrophy. Neurology 2012;79(16):1662-1670.
Martin A, et al. Toward a better understanding of brain lesions during metachromatic leukodystrophy evolution. AJNR Am J Neuroradiol 2012;33(9):1731-1739.
5 A 9-year-old boy with acute onset of headache, vomiting, impaired visual acuity, and focal seizure.
|
What is the most likely diagnosis?
A. Metachromatic leukodystrophy
B. X-linked adrenoleukodystrophy
C. Canavan disease
D. MELAS
E. Alexander disease
View Answer
5 Answer D. MELAS. Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes.
|
Imaging Findings: A. Axial T2-weighted MR image shows a T2 hyperintense signal in the cortical and subcortical white matter within the left occipital and posterior parietal lobes that are not restricted to a specific arterial distribution.
B. Axial diffusion image shows matching-restricted diffusion. C. Follow-up axial FLAIR at 14 years of age shows multiple additional hyperintense lesions within the cortex and white matter. D. Follow-up sagittal T1-weighted MR image at 14 years of age shows cerebellar atrophy.
Discussion: Mitochondrial encephalomyopathy with lactic acidosis and strokelike episodes (MELAS) is a maternally inherited mitochondrial disorder that is caused by point mutations within the mitochondrial DNA affecting transfer RNA genes. The A3243G mutation in the tRNAleu(uur) gene of the mitochondrial DNA is responsible for about 80% of MELAS cases.
Most patients become symptomatic before the age of 40 years. The early development is usually normal, followed by the onset of exercise intolerance, stroke-like episodes, seizures, and dementia. Almost all patients have lactic acidosis and had ragged red fibers in skeletal muscle biopsy specimens. Recurrent migraine-like headaches preceded by nausea and vomiting, hearing loss, short stature, learning difficulties, hemiparesis and hemianopia, and limb weakness are additional common features.
In the acute phase, MRI typically shows swelling and T2/FLAIR hyperintense signal and matching-restricted diffusion in the affected areas, which usually involve the parietal and occipital cortex and subcortical white matter as well as the basal ganglia. Cerebellar atrophy typically develops over time. Follow-up MR studies may show additional new lesions. The lesions are not restricted to a specific arterial distribution, and single lesions often cross vascular boundaries. 1H-MR spectroscopy shows high lactate in affected areas of the brain.
Differential Diagnosis: Ischemic lesions from embolic or thrombotic infarction are typically not restricted to a specific arterial distribution and do not cross vascular boundaries. Urgent administration of nitric oxide precursors (e.g., arginine) in patients with MELAS ameliorates the clinical symptoms associated with stroke-like episodes.
References: El-Hattab AW, et al. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab 2015;116(1-2):4-12.
Ito H, et al. Neuroimaging of stroke-like episodes in MELAS. Brain Dev 2011;33(4):283-288.
Tschampa HJ, et al. Neuroimaging characteristics in mitochondrial encephalopathies associated with the m.3243A>G MTTL1 mutation. J Neurol 2013;260(4):1071-1080.
6 A 6-month-old child with hypotonia, poor head control, loss of milestones, and macrocephaly.
|
What is the most likely diagnosis?
A. Metachromatic leukodystrophy
B. X-linked adrenoleukodystrophy
C. Canavan disease
D. MELAS
E. Alexander disease
View Answer
6 Answer C. Canavan disease.
|
Imaging Findings: A. Axial T2-weighted MR image shows diffuse T2 hyperintense signal of the white matter, absence of any myelination in the internal capsules, and abnormal T2 hyperintense signal of the globi pallidi and thalami. B. 1H-magnetic resonance spectroscopy shows a marked increase in N-acetylaspartate.
Discussion: Canavan disease or spongiform leukodystrophy is an autosomal recessive disorder caused by a deficiency of aspartoacylase. This enzyme is important for the hydrolysis of N-acetylaspartate (NAA).
Children with Canavan disease are typically normal for the first 1 to 2 months of life. Then, they develop hypotonia, poor head control, poor contact, seizures, macrocephaly, and loss of early milestones. In most patients, neurologic abnormalities and macrocephaly are apparent by 6 months of age. Later features of the disorder may include spasticity, opisthotonus, and decerebrate or decorticate posturing.
MR images usually reveal diffuse, symmetric T1 hypointense, and T2/FLAIR hyperintense abnormalities of the cerebral white matter without any focal predominance. The subcortical white matter is preferentially affected early in the course of the disease. The globi pallidi are nearly always affected with sparing of the adjacent putamen. Thalami are frequently involved. The dentate nuclei may also be affected. Proton spectroscopy shows increased NAA peak: this finding is strongly suggestive of Canavan disease and is already present when the rest of the MRI is still normal (neonatal age).
Differential Diagnosis: Preferential involvement of the subcortical white matter may be also seen in L-2-hydroxy glutaricaciduria, Kearns-Sayre disease, propionic acidemia, and urea cycle disorders. Canavan disease occurs more frequently among patients of Ashkenazi Jewish descent.
References: Janson CG, et al. Natural history of Canavan disease revealed by proton magnetic resonance spectroscopy (1H-MRS) and diffusion-weighted MRI. Neuropediatrics 2006;37(4):209-221.
Kumar S, et al. Canavan disease: a white matter disorder. Ment Retard Dev Disabil Res Rev 2006;12(2):157-165.
Van der Knaap MS. Magnetic resonance in childhood white-matter disorders. Dev Med Child Neurol 2001;43:705-712.
7 A 15-year-old girl presents with coarse tremors and dysarthric speech. Images are presented from an MRI scan.
|
7a The most likely diagnosis based on imaging findings is:
A. Hypoxia
B. Carbon monoxide poisoning
C. Renal failure
D. Wilson disease
7b Besides the liver, which organ system involvement is characteristic of Wilson disease?
A. Heart
B. Spleen
C. Eye
D. Kidney
View Answer
7b Answer C. Eye.
|
Imaging Findings: Axial T2-weighted images demonstrate increased signal in the bilateral putamen, caudate nucleus, dorsal midbrain, and anterior pons.
Discussion: Wilson disease (WD) is an inborn error of copper metabolism caused by a mutation to the copper-transporting gene ATP7B. The disease has an autosomal recessive mode of inheritance and is characterized by excessive copper deposition, predominantly in the liver and brain. Diagnosis of the condition depends primarily on clinical features, biochemical parameters, and the presence of the Kayser-Fleischer ring along the periphery of the cornea. Patients with hepatic WD usually present in late childhood or adolescence. The mean age of onset of “neurologic WD” is in the second to third decade. Patients commonly present with extrapyramidal, cerebellar, and cerebral-related symptoms in either a subacute or a chronic fashion. MR imaging abnormality in patients with Wilson disease can be related to hepatic dysfunction manifested by increased T1 signal intensity in the globus pallidus, putamen, and mesencephalon. Increased T2 signal in the putamen, thalami, and brainstem reflects copper deposition in brain tissue, which can result in edema, necrosis, and spongiform degeneration. The midbrain can show “face of the giant panda,” and dorsal pontine abnormalities resemble the “face of a panda cub.”
Signal abnormalities vary according to the stage of the disease and can be reversible with therapy in the early stages.
Differential Diagnosis: Toxic and metabolic etiologies like carbon monoxide poisoning, nitrous oxide toxicity, methanol poisoning, cyanide poisoning, metronidazole poisoning, hypoglycemia, hypoxia, CJD, Leigh disease, or viral encephalitis. Age and associated clinical findings are usually helpful in establishing a diagnosis.
References: Das SK, Ray K. Wilson’s disease: an update. Nat Clin Pract Neurol 2006;2(9):482-493.
Review. PubMed PMID: 16932613.
Singh P, Ahluwalia A, Saggar K, et al. Wilson’s disease: MRI features. J Pediatr Neurosci 2011; 6(1):27-28. doi: 10.4103/1817-1745.84402.
8 A 10-year-old girl with progressive loss of developmental milestones, macrocephaly, and swallowing disorder; unremarkable until the age of 5 years.
|
What is the most likely diagnosis?
A. Metachromatic leukodystrophy
B. X-linked adrenoleukodystrophy
C. Canavan disease
D. MELAS
E. Alexander disease
View Answer
8 Answer E. Alexander disease.
|
Imaging Findings: A-D. Axial T2-weighted MR images show symmetric T2 hyperintense signal of the frontal white matter and posterior limb of the internal capsule. In addition, multifocal nodular brainstem T2 hyperintense abnormalities and a T2 hypointense periventricular rim are noted. E and F. Axial contrast-enhanced T1-weighted MR images show enhancement within the periventricular regions and brainstem.
Discussion: Alexander disease is an autosomal dominant disease caused by pathogenic variants within the gene encoding the glial fibrillary acidic protein (GFAP).
Alexander disease may present with two predominant clinical phenotypes: the infantile and the juvenile or adult forms. Children with the infantile form may present with seizures, pyramidal and sometimes extrapyramidal signs, and loss of motor and cognitive milestones. Macrocephaly is an additional characteristic feature. Patients with the juvenile or adult form may present with predominant motor dysfunction, often with progressive gait disturbance or fine motor difficulties, clinically evident spasticity, and bulbar symptoms (e.g., dysphonia, dysphagia, and palatal myoclonus).
Van der Knaap et al. have described five MRI criteria by which the diagnosis of infantile Alexander disease can be made: (a) extensive cerebral white matter changes (T1 hypointensity and T2 hyperintensity) with frontal predominance, (b) a periventricular rim with hyperintense signal on T1-weighted images and hypointense signal on T2-weighted images, (c) abnormalities of the basal ganglia (particularly the caudate heads and anterior putamina) and thalami, (d) brainstem abnormalities, and (e) contrast enhancement of periventricular regions and lower brainstem. Patients with the juvenile or adult form of Alexander disease typically have regions of T1 hypointense and T2 hyperintense signal within the medulla, pons, and middle cerebellar peduncles that are intensely enhancing after intravenous injection of contrast agents.
Differential Diagnosis: The typical pattern of the infantile form of Alexander disease is quite specific and dissimilar from pattern observed in other white matter diseases. Predominant involvement of the frontal white matter may be seen in X-linked adrenoleukodystrophy. In patients with congenital muscular dystrophy because of LAMA2 pathogenic variants, extensive white matter involvement with relative sparing of the occipital white matter may be seen, but the basal ganglia and thalami are typically spared. Alexander disease should be considered in any patient with tumor-like lesion within the posterior fossa.
References: Rodriguez D, et al. Infantile Alexander disease: spectrum of GFAP mutations and genotype-phenotype correlation. Am J Hum Genet 2001;69:1134-1140.
Van der Knaap MS, et al. Unusual variants of Alexander’s disease. Ann Neurol 2005;57:327-338.
Van Poppel K, et al. Alexander disease: an important mimicker of focal brainstem glioma. Pediatr Blood Cancer 2009;53:1355-1356.
9 A 6-year-old presents with vision problems and skin discolorations.
|
9a What is the most likely diagnosis?
A. PHACES syndrome
B. NF1
C. NF2
D. Metastatic disease
View Answer
9a Answer B. NF1. There are FLAIR hyperintense lesions within the pons/brachium pontis and medial temporal lobes along with a mass lesion within the right greater than left optic chiasm. In a young patient with skin discolorations, NF1 is the most likely diagnosis. PHACES is a syndrome with Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, Eye abnormalities, and Sternal (ventral) defects.
9b In regard to optic pathway glioma prognosis, which statement is correct?
A. NF1-related optic pathway glioma has the best prognosis.
B. Childhood sporadic optic pathway glioma not associated with NF1 has the best prognosis.
C. Adult optic pathway glioma has the best prognosis.
D. All of the above optic pathway gliomas have the same prognosis.
View Answer
9b Answer A. NF1-related optic pathway glioma has the best prognosis. NF1-related optic pathway glioma has the best prognosis followed by childhood sporadic optic pathway gliomas. NF1-related optic pathway gliomas are often pilocytic astrocytomas WHO 1, whereas adult optic pathway gliomas are usually glioblastomas and have the worst prognosis.
|
Imaging Findings: Figures A and B show nonspecific FLAIR hyperintense signal within the pons, brachium pontis, and medial temporal lobes. Figures C and D show a mass lesion centered in the right greater than left optic chiasm compatible with an optic pathway glioma. In a child with FLAIR hyperintense lesions, optic pathway glioma, and skin discolorations (café au lait spots), NF1 is the most likely diagnosis.
Discussion: Neurofibromatosis 1 is an autosomal dominant inherited disease from a mutation in the NF1 gene located on chromosome 17. Although there is virtually 100% penetrance of the disease, the phenotypic expression can be quite variable with some expressing predominantly peripheral lesions, others with paraspinal abnormalities, and others with the intracranial findings. NF1 can be diagnosed with 2 or more of the following: 6 or more café au lait spots, 2 or more neurofibromas, or 1 plexiform neurofibroma, axillary/inguinal freckling, optic glioma, distinctive osseous lesion (sphenoid wing dysplasia, bowing of long bone with or without pseudoarthrosis), and first-degree relative with NF1.
CNS imaging characteristics of NF1 includes nonenhancing hyperintense T2/FLAIR lesions. These lesions are often found in the deep gray matter, hippocampi, brainstem, and cerebellum with little to no mass effect. They can increase in size and number as the child grows but tend to diminish in teenage years and resolve by adulthood. Optic pathway gliomas in NF1 can occur anywhere from the optic nerve through the optic radiations. Plexiform neurofibromas course along peripheral nerves and are hyperintense on T2-weighted imaging with central hypointensity—“target sign.” Sphenoid wing dysplasia manifests by distortion or absence of the lateral orbital wall and often associated with an orbital plexiform neurofibroma. Rarely, NF1 patients can have a vascular dysplasia with moyamoya-like arteriopathy with vessel narrowing and collateral formation.
References: Borofsky S, et al. Neurofibromatosis: types 1 and 2. AJNR Am J Neuroradiol 2013;34: 2250-2251.
Czyzyk E, et al. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol 2003;18(7):471-478.
Ferner RE, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet 2007;44(2):81-88.
10a A 6-year-old girl presents with progressive dystonia and dysphagia. An MRI was performed.
|
What is the most likely diagnosis based on the images?
A. Wilson disease
B. PKAN (pantothenate kinase-associated neurodegeneration also known as Hallervorden-Spatz syndrome)
C. Alexander disease
D. Canavan disease
View Answer
10a Answer B. PKAN (pantothenate kinase-associated neurodegeneration also known as Hallervorden-Spatz syndrome).
10b Regarding Hallervorden-Spatz disease, which of the following is responsible for the hypointensity in the globus pallidus?
A. Deposition of manganese
B. Deposition of calcium
C. Deposition of iron
D. Deposition of copper
View Answer




10b Answer C. Deposition of iron.
|
Imaging Findings: Axial T2-weighted (A) and axial gradient echo images (B) demonstrate hypointense signal in the globus pallidus with strong susceptibility effect on the GRE image.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree