, Yves Etienne2 and Maurice Niddam3
(1)
Faculté de Médecine, Marseille, France
(2)
Unité de Médecine Légale, Hôpital de la Timone, Marseille, France
(3)
Unité SAMU 13, Centre 15, Hôpital de la Timone, Marseille, France
The anatomical study of the relationship of the amygdaloid nuclear complex and its connection routes has given us already a very good idea of the structures with which this complex can exchange information and various stimuli, whether it receives their axons (carriers of the centripetal impulses “inputs”) or it projects its own (carriers of centrifugal impulses or “outputs”) to the said structures.
We must now make recourse to neuroscience to know from where the impulses entering the amygdala are coming and towards which structures are moving the impulses emerging from the amygdala that only immunohistochemical techniques and anterograde or retrograde tracing methods allow us to understand precisely. It is the results of studies using these methods we will review in this chapter, which therefore will be rather a review of the literature.
We shall examine successively the afferent nerve fibres in the amygdaloid complex and efferent fibres coming from this complex. The afferent fibres guide the centripetal amygdalopetal impulses, and the efferent fibres guide centrifugal amygdalofugal impulses.
8.1 Inputs
We distinguish between the afferent fibres, the fibres coming from the olfactory tract, the fibres from the hypothalamus, the fibres from the thalamus, the fibres from the basal part of the telencephalon, the fibres from the brainstem, the fibres coming from the hippocampus and the fibres from the neocortex.
8.1.1 Fibres from the Olfactory Tract
They arise from the olfactory bulb itself, the anterior olfactory nucleus (located in the groove occupied by the olfactory tract) and the lateral olfactory stria (Y Soudry et al. 2011). Let us recall that at the macroscopic level, this stria reaches the prepiriform cortex at the limen (several fibres reaching the primary olfactory cortex located in the insular angle in front of the agranular cortex of the insula) and gives a dissectible large contingent (see Fig. 7.1), detaching almost perpendicularly to reach the convexity which raises the cortical nucleus of the amygdala, at the level of the periamygdaloid anterior cortex. Research carried out by DL Rosene and CW Van Hoesen in 1977 and then by ST Carmichael et al. in 1994 that used, in macaques, a combination of anterograde and retrograde axonal tracers to study these inputs confirmed that the fibres of olfactory origin come to the cortical nucleus of the amygdala and the periamygdaloid cortex (semilunar gyrus and gyrus ambiens). Otherwise, the prepiriform cortex which also receives a macroscopic quota from lateral olfactory stria projects at the baso-lateral nucleus of the amygdala (TPS Powell et al. 1965). The olfactory bulb and prepiriform cortex also target the three parts (dorsal, ventral and ventromedial) of the entorhinal cortex (JE Krettek and JL Price 1977, online 2004) whose deep layers project to the amygdala (M Hoïstad and H Barbas 2008).
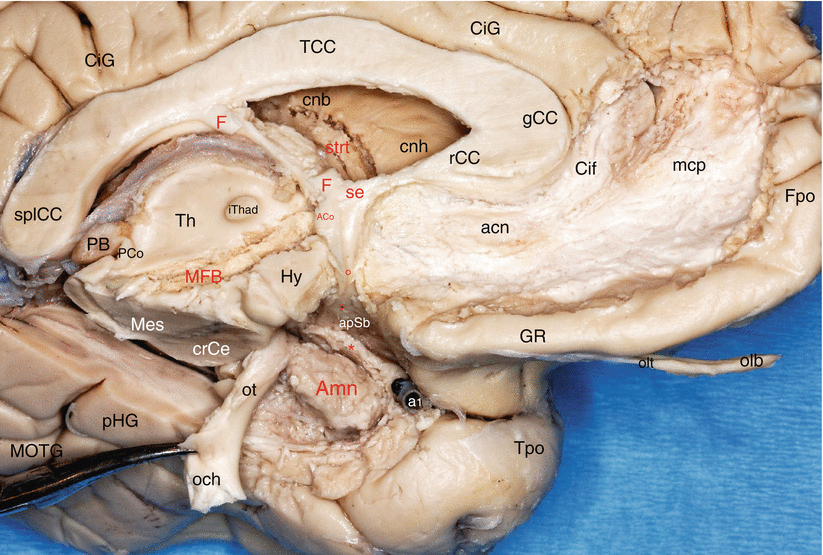

Fig. 8.1
The relations of Amn with the tracts and fasciculi converging to the septal area (components of the “mesolimbic system”). Medial dissection of the left hemisphere. acn accumbens nucleus, ACo anterior commissure, Amn amygdaloid nuclear complex, apSb anterior perforated substance, Cif cingulate fasciculus, CiG cingulate gyrus, cnb caudate nucleus body, cnh caudate nucleus head, crCe crus cerebri, F fornix, Fpo frontal pole, gCC genu of the corpus callosum, GR gyrus rectus, Hy hypothalamus, iThad interthalamic adhesion, mcp mesocortical pathway, MFB medial forebrain bundle, MOTG medial occipitotemporal gyrus, och optic chiasm, olb olfactory bulb, olt olfactory tract, ot optic tract (moved down and backwards by a forceps), PB pineal body, PCo posterior commissure, pHG parahippocampal gyrus, se septum, splCC splenium of the corpus callosum, strt stria terminalis, Th thalamus, Tpo temporal pole, red asterisk peri-amygdalar cortex (pAm cx), little red circles they locate the diagonal band, a1 anterior cerebral artery. Note: the oblique route of the MFB through the hypothalamus and the converging directions of the white fibre tracts to the septal area
8.1.2 Fibres from the Hypothalamus
They have been studied by JP Aggleton et al. in 1980. They mainly come from the ventromedial hypothalamus and from other hypothalamic nuclei (including paraventricular and arcuate nuclei and the lateral hypothalamus) which are also involved in these inputs that go to the medial and central nuclei of the amygdala, leading visceral inputs. Note also that the anatomical dissection has already shown us bundles of nerve fibres connecting the lateral hypothalamus to the central nucleus of the amygdala (see Fig. 7.13).
8.1.3 Fibres from the Thalamus
The thalamic inputs come from the dorsal and ventral regions of the thalamus. They were identified in 1979 by OP Ottersen and Y Ben-Ari in cats and in 1980 by WR Mehler in monkeys and also the same year by M Norita and K Kawamura (1980) and by JP Aggleton et al., and then confirmed in 1990 by JE LeDoux et al. (1990b).
Dorsal inputs
For JP Aggleton et al. (1980) most of the afferent projections come from the rostral midline thalamic nuclei when no projection is found from the medio-dorsal nucleus. In 1982 Y Ben-Ari and E Tremblay specify that inputs coming from the central portion of the thalamus (intralaminar nuclei, in particular from the parafascicular nucleus) and the midline nuclear complex go to the central nucleus of the amygdala designated in 1979 by OP Ottersen and Y Ben-Ari, as “ The major termination area of the thalamo-amygdaloid projections”. DX Zhang, EH Bertram in 2002, obtained similar results using electrical stimulation methods of the median thalamus, but the excitation of the amygdala is associated with activation of the entorhinal cortex. They conclude that the midline thalamic nuclei play “a significant role in limbic physiology and may serve to synchronize the activity in this system”.
The thalamic nuclei which receive inputs from the spinothalamic tract (JE LeDoux 1987) project to the baso-medial nucleus (JE LeDoux et al. 1990a).
Inputs coming from the parvocellular part of the VPM, the ventral postero-medial nucleus (nucleus that transmits the visceral taste information), go to the lateral nucleus of the amygdala.
Inputs coming from the medial division of the medial geniculate nucleus, the posterior intralaminar nucleus or the posterior medial complex also go to the lateral nucleus of the amygdala (JM Edeline and NM Weinberger 1992). Thus, during fear conditioned experiments, auditory stimuli (coming from the medial division of the medial geniculate body) go to the lateral nucleus of the amygdala, while contextual stimuli from the hippocampus go to the baso-lateral and baso-medial nuclei (NS Canteras and LN Swanson 1992; JE LeDoux 2000).
Ventral inputs
The projections from the ventral nuclei of the thalamus go to the cortical, medial, central and lateral nuclei of the amygdala.
8.1.4 Fibres from the Basal Part of the Telencephalon
They have also been the subject of much research, particularly in 1980, in the rat (OP Ottersen) and in the monkey (M Norita et al.; WR Melher; JP Aggleton et al.) and in 2000 (K Semba et al.). Thus, Aggleton et al. who practised in macaques macaca mulatta, fluorescent markings with the enzyme horseradish peroxidase, could highlight inputs coming from the BST and the nucleus of the horizontal part of the diagonal band and ending at the medial and central nuclei of the amygdala. They also observed inputs from the innominate substance reaching the basal nuclei of the amygdala: These afferent fibres belong to large cholinergic neurons, which corresponds to the area in which is found the basal nucleus of Meynert. Other inputs from the ventral pallidum project to the lateral and baso-lateral nuclei of the amygdala (OP Ottersen 1980).
8.1.5 Afferent Fibres from the Brainstem
As demonstrated by the works of WR Mehler (1980), M Norita and K Kawamura (1980), JP Aggleton et al. (1980), OP Ottersen and Y Ben-Ari (1979), OP Ottersen (1981) and J Ciriello et al. (1994), all brainstem neurons send axons to the central nucleus of the amygdala which they approach through the ventral pathway. This is the case of neurons in the periaqueductal grey matter, the pars compacta of the substantia nigra, the ventral tegmental area, nuclei of the medulla oblongata (locus coeruleus, dorsal raphe nucleus, lateral parabrachial nucleus, nuclei of the solitary tract, interpeduncular nucleus) and the ventrolateral nucleus of the spinal cord. According to J Hanaway et al. (2001), all these fibres borrow in their ascending path, the medial forebrain bundle and dorsal longitudinal fasciculus (see Figs. 7.21 and 8.1).
The lateral parabrachial nucleus provides the largest contingent of these afferent fibres. It is involved in the severe, disabling tinnitus which results from interactions between auditory neural sites (cochlear and vestibular) and non auditory neural sites (parabrachial nuclei). These nuclei project the aberrant auditory stimuli towards the central nucleus of the amygdala. This one transfers its emotional responses to the insula which transforms them into conscious emotional feelings. We can see on fMRI the activations of the amygdala and the insula during the severe tinnitus and the regression of these activations when tinnitus was treated with high-frequency acoustic treatment (ML Lenhardt et al. 2007).
This is also from the parabrachial area and directly from the spinal cord that the central nucleus of the amygdala can receive nociceptive inputs (R Burstein and S Potrebic 1993). Since as in the amygdala, the nucleus is the effector of the lateral and basal nuclei of the amygdala, the messages transmitted to it by these nuclei can be modified by nociceptive inputs it receives, which can generate nociceptive outputs to the target organs.
The fibres come from the dorsal raphe nucleus, and fibres of the locus coeruleus (which are noradrenergic fibres) require not only the ventral pathway but also the terminal stria. As for inputs coming from substantia nigra and the ventral tegmental area, they explain the abundant dopaminergic innervation of the central nucleus (Y Ben-Ari 1981).
8.1.6 Hippocampal Inputs
The work of DL Rosene, GW Van Hoesen (1977) and RE Saunders et al. (1988) in rhesus monkeys has clarified that these inputs come from CA1 from the prosubiculum and essentially go to the baso-medial nucleus and the ventral cortical nucleus. The lateral and central nuclei also receive hippocampal inputs.
In 2000, the Finnish team of A Pitkänen et al., which has made anterograde and retrograde studies in rats, confirmed these data stating that the inputs to the amygdala come from the rostral half of the entorhinal cortex, from CA1, from the subiculum and from areas 35 and 36 of the perirhinal cortex. The nuclei that receive these inputs are the lateral, baso-lateral, baso-medial, central nuclei as well as the amygdalo-hippocampal area.
In 2008, M Höistad and H Barbas reviewed the temporal projections to the amygdala from the medial part of the temporal pole, the entorhinal and perirhinal areas, the granular and the dysgranular insula (the densest connections) and the agranular insula and the parahippocampal region (scattered connections (see for structure of the insula, Fig. 8.2)). They also confirmed that there are feedback connections between the entorhinal cortex, which projects from its deep layers to the amygdala and receives back projections in layers II–III, which ultimately target the hippocampus. This pathway could explain “how the amygdala can attach emotional value to environmental stimuli, participate in the sequence of information processing of emotions and modulate the formation of emotional memories”.
8.1.7 Neocortical Inputs
They are particularly important and come from different associative cortical areas. They project for most to the baso-lateral nuclear group of the amygdala, which plays a major role for the receipt of inputs. GW Van Hoesen in 1981, L Stefanacci, DG Amaral in 2002 and JE LeDoux et al. (1990b) showed that the auditory inputs come from the anterior half of the superior temporal gyrus, that is to say, the rostral part of area 22 (extension of the lower section of the posterior long insular gyri or secondary acoustic area AII).
MJ Webster et al., in 1991 and JE LeDoux et al., in 1990a, have shown that inputs from the associative visual cortex come from the lower part of the temporal lobe, that is to say, areas 20 and 21.
We must also consider the very important role of the inferior longitudinal fasciculus (Fig. 7.21) which directly brings to amygdala the visual stimuli. RM Bauer, in 1982, reported the case of a patient whose cranial trauma had caused temporal haemorrhages responsible for the bilateral deterioration of inferior longitudinal fasciculus. This patient had lost any emotion aroused by a visual substratum and regretted having been brought to cancel a subscription of the magazine Playboy whose diligent reader he was before his accident and for which he had no more, from now on, any interest.
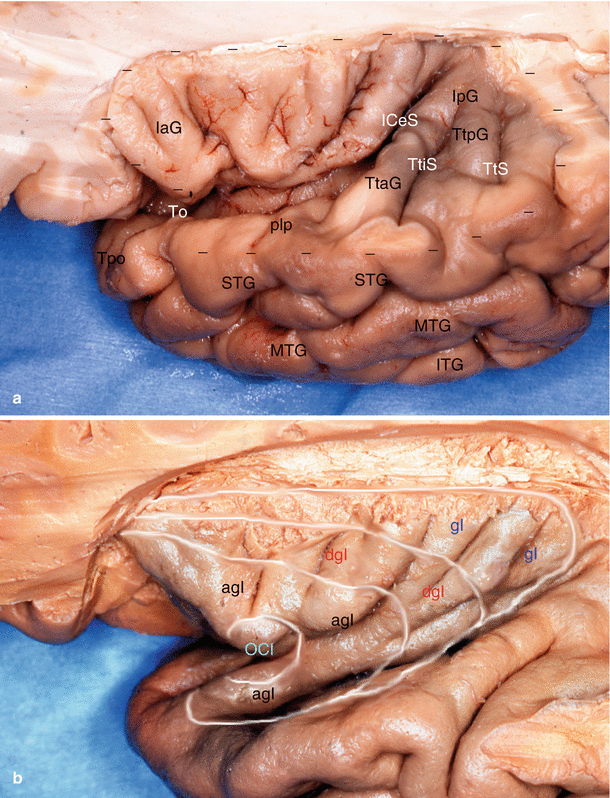

Fig. 8.2
An important relation of the amygdala: the insular cortex. (a) Supero-lateral view of the left human insula: descriptive anatomy. (b) Cortical structure of the insula according to MM Mesulam and EJ Mulson. agI agranular insula, dgI dysgranular insula, gI granular insula, IaG insular anterior gyri (gyri breves), ICeS insular central sulcus, IpG insular posterior gyri (gyri longi), ITG inferior temporal gyrus, MTG middle temporal gyrus, OCI primary olfactory cortex of insula, plp planum polare, STG superior temporal gyrus, To temporal opercule, Tpo temporal pole, TtAG temporal transverse anterior gyrus, TtiS temporal transverse intermedium sulcus, TtPG temporal transverse posterior gyrus, TtS temporal transverse sulcus. The dashed line follows the ilcSI (inferior limiting circular sulcus of insula of Reil)
Note that we have seen in terms of macroscopic anatomy the bundles of fibres that connect the amygdala to the rostral part of each temporal gyrus, conducting resections of the successive gyri from top to bottom of the temporal lobe.
AG Herzog, GW Van Hoesen (1976) and then BH Turner et al. in 1980 and L Stefanacci and DG Amaral in 2000, showed showed that inputs from the multisensory association areas come from the rostral portion of the perirhinal cortex (areas 35 and 36), the cortex forming the dorsal bank of the superior temporal sulcus, the entorhinal cortex, the superior temporal gyrus, the parahippocampal cortex and the temporopolar area (area 38). Let us recall again that the macroscopic study showed us the obvious and important connections between the amygdala and the temporal pole (see Fig. 5.2).
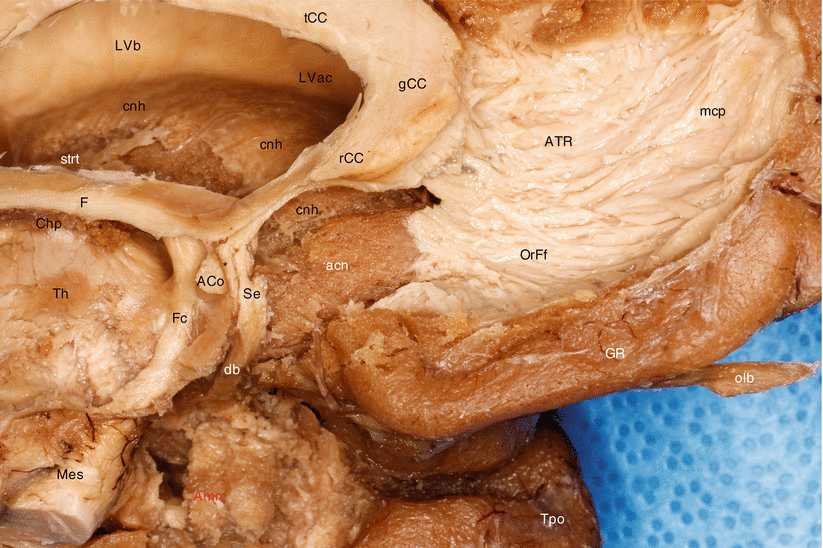

Fig. 8.3
Relationships of the amygdala with the nucleus accumbens septi (dissection of the medial side of the left brain hemisphere). ACo anterior commissure, acn accumbens nucleus, Amn amygdaloid nuclear complex, ATR anterior thalamic radiations, Chpl choroid plexus, cnb caudate nucleus body, cnh caudate nucleus head, db diagonal band, F fornix, Fc column of fornix, gCC genu of the corpus callosum, GR gyrus rectus, LVac lateral ventricle, anterior cornu, LVb lateral ventricle body, mcp mesocortical pathway of MacLean (limbic forebrain–midbrain continuum of Nieuwenhuys), Mes mesencephalon, olb olfactory bulb, OrFf orbitofrontal fibres, rCC rostrum of the corpus callosum, Se septum, strt stria terminalis (note the branch to the caudate nucleus), tCC truncus of the corpus callosum, Th thalamus, Tpo temporal pole, black asterisk prehippocampal rudiment
EJ Mufson et al. (1981) studied inputs involved in gustatory and visceral functions: They come from anterior insular areas agranular areas (Fig. 8.2) that themselves receive impulses from the ventral postero-superior and ventral postero-inferior thalamic nuclei and from the parvicellular division of the ventral postero-medial nucleus of the thalamus. The agranular and dysgranular insular areas project to the lateral nucleus of the amygdala (L Stefanacci and DG Amaral 2000).
MM Mesulam, EJ Mufson in 1985; L Stefanacci, DG Amaral in 2002, studied the posterior part of the insula (Fig. 8.2): it contains a somatosensory association area (granular area), postero-superior, which is connected with the adjacent second sensory area (SmII) (located on the upper lip of the posterior segment of the lateral sulcus) which is involved in the process of nociceptive information.
In a more recent study (2008), M Höistad and H Barbas showed that the medial temporal pole, the entorhinal and perirhinal areas and the agranular and dysgranular parts of the insula have the densest connections with the amygdala, while the lateral temporal pole, the granular insula and the parahippocampal region have sparser connections.
All these inputs coming from the temporal regions (anterior superior temporal, anterior middle temporal and inferior temporal gyri, medial and lateral parts of the temporal pole) and from the insula go mainly to the lateral nucleus of the amygdala and little or not all to other nuclei (L Stefanacci and DG Amaral 2000). The temporal pole is the only one to also target the baso-medial nucleus (AG Herzog and GW Van Hoesen 1976).
We also know now thanks to experiments on conditioned fear (JE LeDoux 2000) that the cortex is not always involved in the immediate analysis of stimuli and the thalamus (receiver of the stimulus to the “medial division of the medial geniculate body”) can directly transfer the stimulus (auditory case) to the lateral nucleus of the amygdala. This has been demonstrated by JS Morris et al. in 1999 by fMRI studies on humans: These authors have observed that during conditioning, amygdala activity changes are correlated with those of the thalamus as is not the case with the cortex which proves the importance of the direct thalamo-amygdaloid trajectory and the direct effector possibilities of the amygdala, in extreme emergency, without cortical modulation! But even in these cases, the stimulus is going to reach the brain analyser controller, some milliseconds later!
Inputs to the amygdala from the frontal lobe are of extreme importance. They are numerous and include the posterior orbitofrontal areas, areas 13 and 14; the medial prefrontal areas, areas 11 and 12 (HT Ghashghaei and H Barbas1 2002; H Barbas 2007); the anterior cingulate area, area 24 (DN Pandya et al. 1973); the prelimbic area, area 32; and the infra-limbic area, area 25. All these inputs from the frontal lobe target the baso-lateral and the baso-medial nuclei of the amygdala. Numerous authors have devoted their research to these inputs, identifying a number of details:
GR Leichnetz, J Astruc (1977) showed that the prefrontal cortex not only projects to the amygdala but also to the substantia innominata and the pallidum.
L Stefanacci and DG Amaral (2000) found found that the orbitofrontal cortex; the prefrontal medial cortex, areas 10, 11, 12, 13, 13a and 14; and the anterior cingulate cortex, areas 25, 24 and 32, mainly target the baso-lateral and baso-medial nuclei and the orbitofrontal cortex and prefrontal medial cortex (but not the anterior cingulated cortex) and also project to the cortical and medial nuclei as well as to the periamygdaloid cortex.
NL Rempel-Clower, in 2007, observed in rats, as H Barbas did the same year in the monkey, that the orbitofrontal cortex also projects to the intercalated masses of the amygdala that are GABAergic and thus exerts inhibitory influences within the amygdala. H Barbas was able to clarify that, in fact, the orbitofrontal posterior cortex projects to the amygdala, through 2 “pathways” that have opposite effects on the central autonomous structures: The first path is the one mentioned above, which leads from the cortex to the intercalated mass and whose activation can ultimately disinhibit central autonomic structures during emotional arousal. The second system innervates the central nucleus of the amygdala that has inhibitory projections to the hypothalamus and autonomous structures of the brainstem and can cause inhibition of central autonomous structures, resulting in an autonomous homeostasis1. HT Ghashghaei and H Barbas in 2002 showed that the lateral prefrontal cortex “which has executive functions” specifically projects to the layer 5 of the orbitofrontal cortex. In 2007, the same authors found that the orbitofrontal neurons sending their axons to the amygdala are from this cortical layer 5. They so demonstrate that also the lateral prefrontal cortex has a controlling role over the inner workings of the amygdala (especially on the posterior half of the amygdala, the intermediate sector of the baso-lateral nucleus and the magnocellular portion of the baso-medial nucleus). It was also in 2007 that M Medalla et al. were able to demonstrate that at the level of the prefrontal cortex, there are two specialised classes of inhibitor neurons, belonging respectively to each of the two pathways mentioned above and expressing the duality and thus the complexity of inhibitory control: the area of inhibitory neurons 32, dedicated to emotional communication, and those in area 10 (see mapping Fig. 7.8), dedicated to working memory functions.
8.2 Outputs
The efferent fibres are not really modelled on inputs even if some of them use the same pathways as these.
We can classify these efferent fibres according to the parts of the brain to which the amygdaloid nuclei will send their outputs. There are also amygdaloid diencephalic projections to the hypothalamus and thalamus, projections to the brainstem, projections to the basal brain, projections to the hippocampus and projections to the neocortex.
Many of these projections borrow the bundles that were studied in terms of connections, and it is from such projections we will begin the study of efferent fibres.
8.2.1 Efferent Fibres Passing via the Stria Terminalis2 (Dorsal Pathway)
1.
Precommissural fibres of the stria have multiple destinations. They lead on the one hand to the septal and paraseptal nuclei (septal nuclei, diagonal band nucleus, nucleus accumbens) and to the rostral nuclei of the hypothalamus (preoptic and anterior). It is recognized that this contingent in the stria originates from the cortico-medial nuclear group of the amygdala.
2.
Commissural fibres allow communication between two stria terminalis and thus between the nuclei of the two amygdalae. As we have seen in dissections, these fibres are positioned exactly in the centre of the anterior commissure (see Fig. 6.7). Before entering the commissure, a part of the contingent reaches the hypothalamic paraventricular, ventromedial and premammillary nuclei.
3.
Post-commissural fibres terminate at the BST and the posterior hypothalamus: The fibres that go to the lateral BST come from the basal and central nuclei of the amygdala. The fibres that go to the medial BST come from the medial and cortical nuclei.
A dorsal contingent of the stria may be attached to post-commissural fibres. It is the thalamic component of the stria which is detached from its dorsal concavity and penetrates the back of the thalamus to reach the medio-dorsal nucleus of this formation. We were able to follow this through dissection, even within the thalamus (see Fig. 6.3). According to JP Aggleton, M Miskin 1984, only a small number of amygdalo-thalamic fibres progress into the terminal stria. We cannot agree to the term “small number” because the stria terminalis contingent destined for the thalamus has always seemed to be of a large diameter (see Fig. 6.3).
4.
The fibres for the striatum use mostly the stria terminalis or its side branches to reach their targets. We have already seen the precommissural branch for the ventral striatum (nucleus accumbens, olfactory tubercle), considered to be the “limbic striatum compartment”. But the stria terminalis also carries axons for the dorsal striatum (caudate and putamen nucleus), “non-limbic compartment” and yet essential. As Y Ben-Ari and E Tremblay (1982) recall since, it provides the amygdala, the possibility of inputs to the motor system and thus of having an effect on the latter. The terminal stria dissection allows to observe some branches for the caudate nucleus, fine for the most part: They touch the edge of this formation enabling axons from the amygdala to reach their striatal target (Fig. 8.3). Specifically the dorsal striatum also receives projections of the anterior baso-lateral nucleus that do not pass through the stria terminalis (GD Petrovich 2001). L Fudge et al. (2002) have shown that fibres which are all taken from the ventral striatum come from neurons of the baso-lateral and baso-medial nuclei of the amygdala. According to FT Russchen et al. (1985b), there is a specificity of origin of the fibres: fibres destined for the nucleus accumbens2 are from the parvicellular part of the basal nuclear group and from the amygdalo-hippocampal area, while projections for the tail of the caudate nucleus come only from the magnocellular part of the basal nuclear group.
We were also able to demonstrate by dissection the connection between the terminal stria and the medullary stria of the thalamus (or stria medullaris). We see perfectly a direct posterior branch detaching from the stria terminalis prior to its development to continue directly with the medullary stria that will rejoin the habenula (see Fig. 6.5), the starting point of the habenulo-interpeduncular tract.
8.2.2 Efferent Fibres Passing Through the Ventral Amygdalofugal Pathway
They are created by the baso-lateral, baso-medial and central nuclei of the amygdala. They immediately use the ventral pathway and then target the structures of various regions: the septal region (particularly to the diagonal band nucleus), the sublenticular region (notably to the basal nucleus of Meynert), the BST, the thalamus and the lateral hypothalamic area.
8.2.2.1 The Amygdalo-thalamic Fibres
Amygdaloid projections go to the medio-dorsal nucleus and to the nucleus reuniens of the thalamus (Y Ben-Ari, E Tremblay 1982; JP Aggleton M Miskin 1984). Those destined for the medio-dorsal magnocellular nucleus essentially use the inferior peduncle of the thalamus and enter the rostral head of the thalamus before bending backwards to reach their target (see Fig. 6.6). They come mostly from the basal group (but almost all amygdaloid nuclei also participate) and terminate in the medial magnocellular portion of the medio-dorsal nucleus (JP Aggleton). Other fibres from the central nucleus of the amygdala project towards the midline thalamic nuclei (JL Price, DG Amaral 1981).
8.2.2.2 The Amygdalo-hypothalamic Fibres
In addition to the fibres connecting directly the amygdala and hypothalamus, which we mentioned earlier, there are many projections from the amygdala to the hypothalamus: the paraventricular nucleus, the dorso-medial nucleus, the perifornical region, the supramammillary and paramammillary nuclei and the lateral hypothalamus (JL Price, DG Amaral 1981). Among them there are the projections to the paraventricular nucleus that have been particularly studied using neuroscience techniques (TS Gray et al. 1989) in monkeys. It is clear from this research that the central nucleus of the amygdala supplies the caudal, lateral and medial parvocellular parts of this hypothalamic nucleus, while the medial nucleus of the amygdala supplies the rostral parvocellular sections of the same nucleus. The authors believe that these anatomical differences explain the amygdaloid modulation of neuroendocrine response to stress factors.
Other fibres coming from the basal nuclei and from the central nucleus of the amygdala will rejoin the medial hypothalamus and the lateral hypothalamic area (WJH Nauta, W Haymaker 1969). These projections show how the amygdala can act on hypothalamic pathways managing specific behaviours (nutritional, sexual, aggressive and defensive).
8.2.2.3 The Amygdalo-basal Fibres And Amygdala-BST Fibres
Amygdala-BST fibres are mainly distributed in three target organs: the diagonal band nucleus, the basal nucleus of Meynert and the BST. On the other hand, a study by FT Russchen et al. in 1985b, in monkeys, using autoradiographic tracing, found few amygdaloid axons reaching the innominate substance and the ventral pallidum. The origin of these amygdaloid fibres was the parvicellular basal nucleus, the caudal section of the magnocellular basal nucleus, the baso-medial magnocellular nucleus and the central nucleus. A new study by YT Cho et al., conducted in 2013, administering a tracer by two-way injection in the amygdala, has not only found a noticeably sharper projections to the striatum but also to the prefrontal and insular cortex, demonstrating at the same time the existence of cortico-amygdalo-striatal pathways.
We shall see successively the amygdalo-diagonal fibres and the fibres of the cortico-amygdalo-striatal pathways, reserving the study of the fibres that project to the nucleus of Meynert and the fibres that connect the amygdala and the BST in the chapter on the extended amygdala because these fibres either belong to the ventral amygdalofugal pathway and in the chapter cited above. Let us recall that in 1981, the links between the basal amygdaloid nuclei and the amygdala-BST were examined by JL Price and DG Amaral in their remarkable study using an autoradiography method for tracing axonal projections (In their publication, one can see images surimposed on whose which we present in the Chap. 11).
The amygdalo-diagonal fibres connect the amygdala to the diagonal band nucleus. These are direct fibres that travel along the so-called horizontal part (actually more or less oblique) of the band to the lower septal region where the discrete thickening sits which corresponds to the nucleus of this band. It should be known that there is a real bond between the infero-medial pole of the amygdala and the lateral area of inflection of the diagonal band at or immediately after the latter has “received” its contingent coming from the limen insulae. This bond is more than just an apposition since there is an exchange of fibres between the amygdaloid periphery and the band’s fibres. Furthermore, the real constitution of the magnocellular diagonal band is complex (see Fig. 7.5) with not only a large part coming from the septum and forming the posterior limit of the anterior perforated material but also two other narrower portions, one coming from the limen and rejoining the band almost at right angles and the other continuing the initial trajectory of the band, curving slightly following a parallel path and overlying the optic tract and ending in the pulvinar, just above the lateral geniculate body (see Figs. 7.2, 7.3, 7.4, and 7.5). Such a constitution suggests amygdala projections which are not yet known towards the limen and probably the insula on the one hand and towards the posterior thalamus, on the other hand.
The fibres of the cortico-amygdalo-striatal pathways
They are better known thanks to the work of YT Cho et al. (2013). They include projections from various prefrontal cortical insular regions towards the baso-lateral and baso-medial nuclei of the amygdala. From these nuclei other neurons target the entire striatum “area extending well beyond the classic ventral striatum”. Depending on the degree of differentiation of the cortical layer from which the fibres reach the amygdala, the authors distinguish three main pathways to the striatum:
A primitive pathway from the least differentiated cortical regions (agranular insula, areas 25 and 32 of the medial prefrontal cortex) and reaching through all nuclei of the amygdala, the ventral striatum.
An intermediate pathway coming from more differentiated insular regions (dysgranular and granular) and areas 24 and 14 of the medial prefrontal cortex, projecting to the rostro-medial and centro-medial areas of the parvocellular portion of the baso-lateral nucleus, the magnocellular portion of the baso-medial nucleus and the magnocellular portion of the baso-lateral nucleus. From these nuclei, efferents pass beyond the classic ventral striatum to the caudo-ventral putamen and the ventromedial caudate nucleus.
A developed pathway, originating in the most differentiated cortex (the entire orbitofrontal cortex and the most developed segment of the medial medio-frontal cortex, medial area 10) only projecting to the magnocellular regions of the baso-lateral and baso-medial nuclei. From these nuclei the efferents project to the caudal part, the dorsolateral body, the knee and the tail of the caudate nucleus. The authors state that they always found this pathway in conjunction with the other two pathways.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree