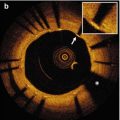
Fig. 7.1
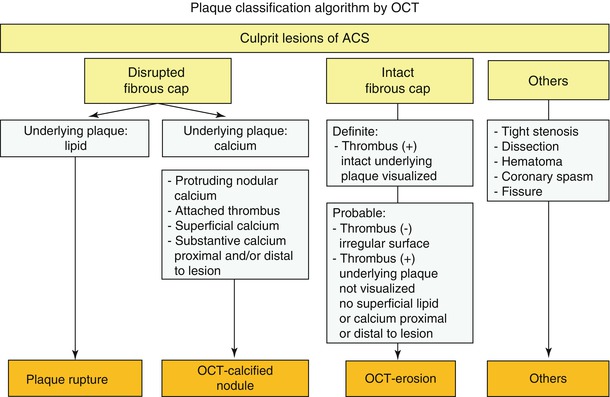
Atherosclerotic lesions with luminal thrombi. (a) Ruptured plaques are thin fibrous cap atheromas with luminal thrombi (Th). These lesions usually have an extensive necrotic core (NC) containing large numbers of cholesterol crystals and a thin fibrous cap (<65 μm) infiltrated by foamy macrophages and a paucity of T lymphocytes. The fibrous cap is thinnest at the site of rupture and consists of a few collagen bundles and rare smooth muscle cells. The luminal thrombus is in communication with the lipid-rich necrotic core. (b, c) Erosions occur over lesions rich in smooth muscle cells and proteoglycans. Luminal thrombus overlies areas lacking surface endothelium. The deep intima of the eroded plaque often shows extracellular lipid pools, but necrotic cores are uncommon; when present, the necrotic core does not communicate with the luminal thrombus. Inflammatory infiltrate is usually absent, but if present, is sparse and consists of macrophages and lymphocytes. (d) Calcified nodules protrude into the lumen through a disrupted thin fibrous cap (FC). There is absence of an endothelium at the site of the thrombus, and inflammatory cells (macrophages, T lymphocytes) are absent (From Virmani et al. [4])
7.2 Classification of Underlying Culprit Lesions in ACS
7.2.1 Evolution of the Paradigm for Atherosclerotic Lesions
The first report on the classification of atherosclerosis was proposed by the American Heart Association (AHA) in 1995 (Table 7.1) [7]. In this classification, atherosclerotic lesions were first divided into two stages (early lesion and advanced lesions), then further categorized into six types of lesions which were designated along an orderly, linear pattern of lesion progression. The perception of rupture of an advanced atherosclerotic lesion as the primary mechanism responsible for ACS was widely accepted. However, this paradigm was challenged by inconsistent findings in two pathological studies [5, 8], which showed that coronary thrombi could arise without rupture. The first study was performed by van der Wal et al. [8], who investigated 20 patients with SCD and found plaque rupture in only 60 % of lesions; the remaining 40 % showed absence of rupture with only superficial erosion which is defined as a thrombus confined to the most luminal portion of a fibrous cap in the absence of fissure or rupture after serial sectioning. The second report was from Virmani’s group [5], who studied the underlying plaque morphology in more than 200 cases of SCD with luminal thrombi in at least one artery with >75 % cross-sectional area luminal narrowing. Only 31 % of lesions in their studies could be defined as plaque rupture, and over one third of lesions did not have evidence of rupture. All these data raised a critical question about the central role of plaque rupture in the previous AHA classification and subsequently led to changes in the classification of atherosclerotic lesions. To better understand the underlying mechanism of ACS, Virmani et al. [4] proposed another plaque morphological classification scheme based on their comprehensive autopsy observation (Fig. 7.2). Their categories included intimal xanthoma, intimal thickening, pathological intimal thickening, fibrous cap atheroma, thin fibrous cap atheroma, calcified nodule, and fibrocalcific plaque. The accumulation of lipid in relation to the formation of fibrous cap which changes over time, as well as thrombosis was taken into account in the classification. More importantly, their classification highlighted the importance of plaque erosion in thrombotic events. Although this classification scheme allows for easier understanding, its practical usability and application in living patients need to be confirmed.
Table 7.1
AHA classification
Terms for atherosclerotic lesions in histological classification | Other terms for the same lesions often based on appearance to the unaided eye | ||
|---|---|---|---|
Type I lesion | Initial lesion |  Fatty dot or streak Fatty dot or streak | 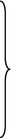 Early lesion Early lesion |
Type II lesion | |||
IIa | Progression-prone type II lesion | ||
IIb | Progression-resistant type II lesion | ||
Type III lesion | Intermediate lesion (pre-atheroma) | ||
Type IV lesion | Atheroma |  Atheromatous plaque, fibrolipid plaque, fibrous plaque Atheromatous plaque, fibrolipid plaque, fibrous plaque |  Advanced lesions, raised lesions Advanced lesions, raised lesions |
Va | Fibroatheroma (type V lesion) | ||
Vb | Calcific lesion (type VII lesion) | Calcified plaque | |
Vc | Fibrotic lesion (type VIII) | Fibrous plaque | |
Type VI lesion | Lesion with surface defect and/or hematoma/hemorrhage and/or thrombotic deposit | Complicated lesion, complicated plaque | |

Fig. 7.2
Simplified scheme for classifying atherosclerotic lesions modified from the current AHA recommendations. The boxed areas represent the seven categories of lesion. Dashed lines are used for two categories because there is controversy over the role that each of them plays in the initial phase of lesion formation, and both “lesions” can exist without progressing to a fibrous cap atheroma (ie, AHA type IV lesion). The processes leading to lesion progression are listed between categories. Lines (solid and dotted, the latter representing the least established processes) depict current concepts of how one category may progress to another, with the thickness of the line representing the strength of the evidence that these events do occur (From Virmani et al. [4])
7.2.2 In vivo Classification of Atherosclerotic Plaque Responsible for ACS
As mentioned above, most reports on lesion classification have been derived from autopsy studies. Not only were the sample sizes of those studies relatively small, but also the study subjects were mostly young, sudden coronary death victims, who could be significantly different from living patients. Seeing these limitations, in vivo classification of coronary atherosclerotic plaques is needed.
Coronary angiography has been used as a gold standard diagnostic modality for the evaluation of patients presenting with ACS. However, an angiogram shows only the luminal outline and is not able to visualize intravascular structure. Although intravascular ultrasound (IVUS) is widely used to evaluate plaque morphology, including plaque burden and remodeling, the resolution is inadequate to characterize subtle changes in the vascular wall. For example, IVUS cannot be used to detect thin fibrous cap, macrophage infiltration, mural thrombus, and irregular or eroded surface, which are key features for characterizing coronary plaque phenotype. In contrast, intravascular optical coherence tomography (OCT), with the unprecedented resolution of 10–15 μm, allows us to detect and quantify specific morphologic changes that are analogous to histological features in some circumstances, including lipid pool, thin fibrous cap, plaque rupture, thrombus, calcification, macrophages, and microchannels (Fig. 7.3) [9–11]. Considering the limitations of OCT and the clinical situations in which patients are being treated with antithrombotics and thrombolysis, we proposed a new set of OCT diagnostic criteria for the culprit lesion classification in patients with ACS (Table 7.2 and Fig. 7.4) [12]. In order to establish this algorithm, it was critical to accurately characterize plaque erosion and calcified nodule in vivo, whose definitions have been well-established by autopsy studies. A few imaging studies have investigated plaque erosion and calcified nodule in patients with ACS [13, 14]. However, the definitions used in those studies were based purely on pathological findings [4] (loss of endothelial cell lines and/or dysfunction of endothelial cells) which are beyond the resolution of OCT. For this reason, we instituted new diagnostic criteria for OCT-erosion and OCT-calcified nodule (OCT-CN) based on pathologic findings and also took into account the limitations of OCT and the differences between living patient and post-mortem examinations (Fig. 7.4). Though this classification is based on only 126 patients presented with ACS, this is the first in vivo study that systematically investigated and established the classification of coronary culprit atherosclerotic plaque in living patients with ACS.
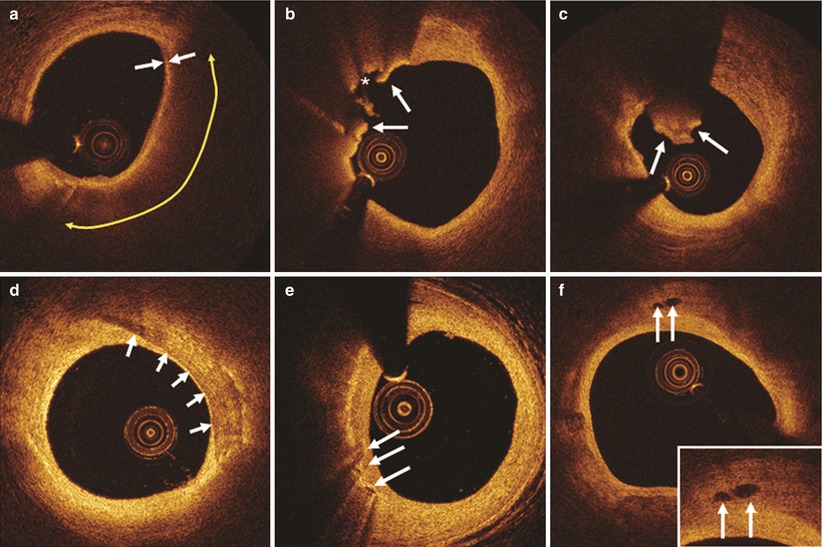

Fig. 7.3
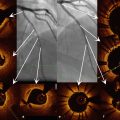
Representative OCT images. (a) Thin-cap fibroatheroma is characterized by thin fibrous cap (≤65 μm, white arrows) and large lipid pool (yellow double-arrow). (b) Plaque rupture (arrows indicate rupture site, asterisk indicates cavity.). (c) Red thrombus is defined as a mass (diameter ≥250 μm) attached to the luminal surface or floating within the lumen, which shows high backscattering with high attenuation (white arrows). (d) Calcification is defined as an area with a signal-poor or heterogeneous region with a sharply delineated border. (e) Macrophage accumulation is defined as signal-rich, distinct or confluent punctate regions that exceed the intensity of background speckle noise (white arrows). (f) Microchannels are defined as signal-poor voids that are sharply delineated in multiple contiguous frames (arrows)
Table 7.2
In vivo classification of culprit lesions in cases of ACS
Lesion type | Description |
|---|---|
Plaque erosion | Intact fibrous cap; with or without luminal thrombosis; underlying plaque can be lipid, fibrous, or intimal thickening; calcification rare |
Definite OCT-erosion | Luminal thrombus; intact fibrous cap; underlying plaque visible |
Probable OCT-erosion | (a) Absence of luminal thrombus; irregular luminal surface; |
(b) Luminal thrombus; attenuation of underlying plaque by thrombus; no superficial lipid or calcification immediately proximal or distal to the site of thrombus | |
Plaque rupture | Lipid plaque with cap disruption; with or without luminal thrombus; cavity may exist |
Calcified nodule | Disrupted fibrous cap; protruding nodular calcification with underlying fibrocalcific plaque; luminal thrombus; superficial calcium; substantive calcium proximal and/or distal to lesion |
7.3 Plaque Rupture
7.3.1 Definition
In a consensus statement, ruptured plaque was defined as plaque with a structural defect or gap in the fibrous cap that separates the lipid-rich necrotic core of a plaque from the flowing blood, thereby exposing the thrombogenic core of the plaque [15]. OCT characterizes plaque rupture by the presence of fibrous cap discontinuity with a clear cavity formed inside a lipid-rich plaque (Fig. 7.5). In contrast to pathology, OCT does not require the presence of overlying thrombus in the diagnosis of this type of lesion in living patients who may have already been treated with antithrombotic or thrombolytic therapy before imaging. Ruptured plaque typically has a large lipid-rich necrotic core (>30 % of the plaque area) and a disrupted thin fibrous cap infiltrated by a large number of macrophages and lymphocytes, and a sparse distribution of SMCs (See Table 7.3). Although some other less-defined terms like fissure and disruption have been used by some investigators, in order to avoid confusion, plaque rupture is recommended for future OCT research work.
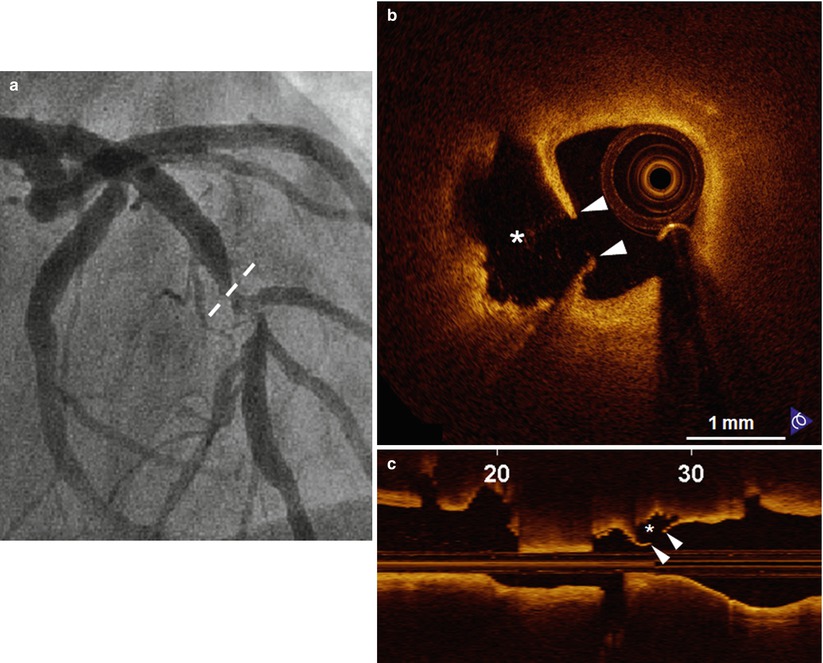

Fig. 7.5
Representative case of “Plaque Rupture”. A 57-year-old man presenting with STEMI was treated with thrombolysis. (a) The coronary angiogram shows the culprit lesion in the mid LAD (dashed line indicates the ruptured site corresponding to B). Plaque rupture is identified on cross-sectional (b) and longitudinal (c) OCT images by the disrupted fibrous-cap (arrowheads) and a cavity (asterisk) formation inside the plaque. STEMI ST-segment elevation myocardial infarction, LAD left anterior descending coronary artery (From Jia et al. [12])
Table 7.3
Features of ruptured plaques
Thrombus |
Large necrotic core (>30 % of plaque) |
Fibrous cap covering the necrotic core |
Thin (thickness usually <65 μm) |
Many macrophages (inflammation) |
Few smooth muscle cells (apoptosis) |
Expansive remodelling preserving the lumen |
Neovascularization from vasa vasorum |
Plaque hemorrhage |
Adventitial/perivascular inflammation |
‘Spotty’ calcification |
7.3.2 Incidence
The incidence of plaque rupture as an underlying pathology of ACS ranged from 50 to 75 % in autopsy studies depends on the population: 75 % in patients with acute myocardial infarction (AMI), 55–65 % in SCD, and 36 % in unstable angina (UA) [4, 6, 16–20]. Based on the most recent data from a worldwide survey (See Table 7.4) including 22 autopsy studies in which 1,847 coronary arteries were studied with the purpose of characterizing the underlying atherosclerotic plaques [21], plaque rupture was the primary cause of coronary thrombi in approximately 73 % of these fatal events, regardless of clinical presentation (myocardial infarction: 79 %; or sudden death: 65 %), age (>60 years: 77 %; <60 years: 64 %), sex (male: 76 %; female: 55 %) and geographic origin (Asia: 81 %; Europe: 72 %; USA: 68 %.) [4, 6, 8, 17, 22–39]. (Fig. 7.6) Several in vivo studies confirmed that plaque rupture is the most common cause of acute coronary events. The very first in vivo OCT study to evaluate culprit lesion morphology in the setting of ACS was conducted by our group [9]: we found the frequency of thin-cap fibroatheroma (TCFA), a precursor of plaque rupture, was significantly higher in ACS, in comparison to stable angina pectoris (SAP) (72 % in AMI, 50 % in UAP, and 20 % in SAP; p = 0.012). Interestingly, the frequency of thrombus and plaque rupture was not significantly different between the groups and generally lower, relative to previous pathological studies [4, 40]. This could be caused by the discrete sampling method used in this study and the time delay between symptom onset and imaging, and antithrombotic therapy before imaging. Following this study, another group from Japan used multimodality imaging including OCT, IVUS, and angioscopy, to assess underlying plaque characteristics [13]. This study highlighted the advantages of OCT for the detection of plaque rupture (73 % versus 40 % versus 43 %, p = 0.021), erosion (23 % versus 0 % versus 3 %, p = 0.003), and thrombus (100 % versus 33 % versus 100 %, p < 0.001), in comparison to IVUS and angioscopy.
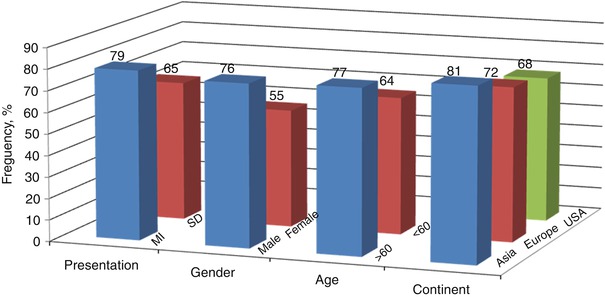
Table 7.4
Plaque rupture underlying 1,345 (73 %) of 1,847 fatal coronary thrombi worldwide
Patients | Age,a years | Cases, n | Rupture, % | Study |
|---|---|---|---|---|
Hospital, – | — | 19 | 19/19 = 100 | Chapman [23] |
Hospital, – | — | 17 | 17/17 = 100 | Constantinides [24] |
Hospital, AMI + SCD | 58 | 40 | 39/40 = 98 | Friedman and Van den Bovenkamp [25] |
Hospital, AMI | 62 | 88 | 71/88 = 81 | Bouch and Montgomery [26] |
Hospital, AMI | 66 | 91 | 68/91 = 75 | Sinapius [27] |
Coroner, SCD | 53 | 20 | 19/20 = 95 | Friedman et al. [28] |
Hospital, AMI | 67 | 76 | 69/76 = 91 | Horie et al. [29] |
Hospital, AMI | 67 | 49 | 40/49 = 82 | Falk [30] |
Coroner, SCD | <65 | 32 | 26/32 = 81 | Tracy et al. [31] |
Med. Exam, SCD | <70 | 61 | 39/61 = 64 | El Fawal et al. [17] |
Hospital, AMI | — | 83 | 52/83 = 63 | Yutani et al. [32] |
Coroner, – | — | 85 | 71/85 = 84 | Richardson et al. [33] |
Hospital, AMI | 63 | 20 | 12/20 = 60 | van der Wal et al. [8] |
Coroner, SCD | — | 202 | 143/202 = 71 | Davies [34] |
Hospital, AMI | 69 | 291 | 218/291 = 75 | Arbustini et al. [6] |
Hospital, AMI | 61 | 61 | 56/61 = 92 | Shi et al. [35] |
Hospital, AMI | 69 | 100 | 81/100 = 81 | Kojima et al. [22] |
Med. Exam, SCD | 48 | 360 | 212/360 = 59 | Virmani et al. [4] |
Med. Exam, AMI + SCD | — | 31 | 26/31 = 84 | Murai et al. [36] |
Hospital, SCD | — | 58 | 34/58 = 59 | Giannoukas andcoworkers [37] |
Hospital, AMI | — | 14 | 10/14 = 71 | Sato et al. [38] |
Coroner, SCD | 54 | 49 | 23/49 = 47 | Subirana et al. [39] |
AMI + SCD | — | 1,847 | 1,345/1,847 = 73 | Worldwide |

Fig. 7.6
Frequency of plaque rupture
7.3.3 Plaque Ruptures in STEMI and Non–ST-segment Elevation Acute Coronary Syndrome
The paradigm proposed by pathologists is that the underlying mechanism is identical between STEMI and non–ST-segment elevation acute coronary syndrome (NSTEACS). However, this is not the case in living patients presenting with ACS. In our clinical study, we investigated the underlying plaque characteristics in over 200 cases with ACS using intravascular OCT [12]. Plaque rupture was found to be responsible for the culprit lesion in 44 % of patients, while 31 % had plaque erosion. Plaque rupture was more frequently observed in patients with STEMI (72 %) than in those with NSTEACS (32 %). Another OCT study by Ino et al. [41] found consistent results in frequency of plaque rupture (70 % versus 47 %, p = 0.033); its precursor lesion-TCFA (78 % versus 49 %, p = 0.008) was also significantly higher in patients with STEMI than in those with NSTEACS. Moreover, they found that patients with STEMI had different ruptured plaque morphologies as compared to those with NSTEACS. Although the minimum lumen area was similar in both groups, the ruptured cavity size was significantly larger in those with STEMI as compared to those with NSTEACS. Lastly, the ruptured plaque of which aperture was open wide against the direction of coronary flow, was more often seen in STEMI compared to NSTEACS (46 % versus 17 %, p = 0.036).
7.3.4 Plaque Rupture and Exertion
Burke et al. [42] compared the frequency and morphology of plaque rupture in 116 men whose deaths occurred at rest to that of 25 men who died during strenuous activity or emotional stress. Plaque rupture was observed in 17 (68 %) of 25 men dying during exertion versus 27 (23 %) of 116 men dying at rest (p < 0.001). The mean minimum thickness of the fibrous cap in exertion-related plaque ruptures was thinner compared with that in rest-related plaque ruptures. Exertion-related plaque rupture was more frequent in the center of the plaque, while rest-related rupture was more frequent in the shoulder of the plaque (Fig. 7.7). However, one OCT study reported opposite results that fibrous cap thickness (FCT) was thinner in rest-onset ACS compared with exertion-triggered ACS (50 μm versus 90 μm, p = 0.002), and the rupture at plaque shoulder was more frequent in the latter (57 % versus 93 %, p = 0.017) (Fig. 7.8) [43]. Furthermore, not only TCFA but also thick cap fibroatheroma (ThCFA) might rupture. The findings from this study suggested that FCT was a more important determinant of plaque instability than the shoulder region where the greatest stress was exerted, and fibrous cap rupture could occur at the site of plaque shoulder even in ThCFA up to 150 μm during exertion.
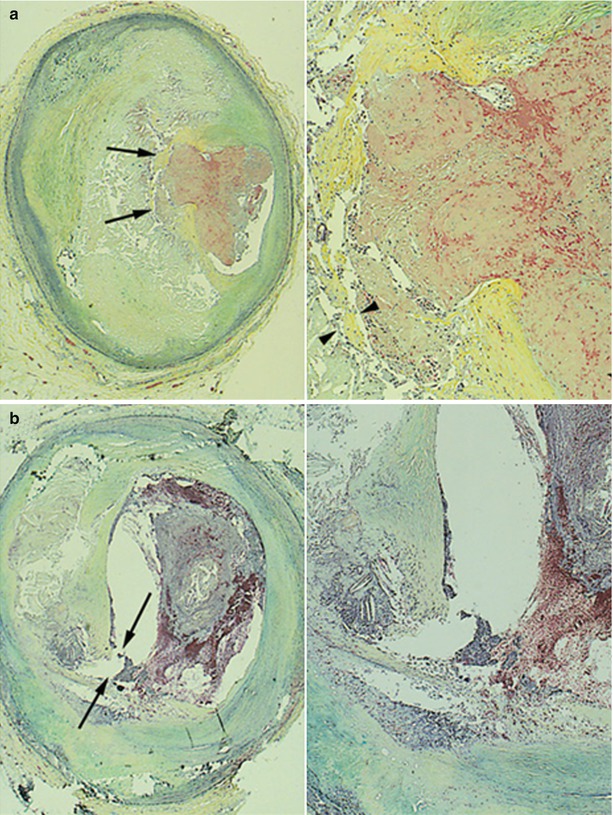

Fig. 7.7




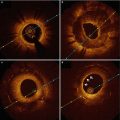
Plaque ruptures in relation to exertion. (a) Plaque Rupture at the Shoulder Region of the Fibrous Cap. A 38-year-old man collapsed suddenly during an altercation. Left, The rupture site is toward the center of the fibrous cap (arrows) (Movat pentachrome, original magnification ×15). Right, A higher magnification demonstrating rupture site with acute thrombus. Note areas of thinning of fibrous cap (arrowheads) (Movat pentachrome, original magnification ×45). (b) Plaque Rupture at the Center of the Fibrous Cap. A 60-year-old man was found dead in bed. Left, The site of rupture is present at the junction of the fibrous cap with the mildly thickened intima of the relatively normal arterial wall (shoulder region) (arrows) (Movat pentachrome, original magnification ×15). Right, A higher magnification of the shoulder area showing rupture site and overlying thrombus (Movat pentachrome, original magnification ×30) (Adapted from Burke et al. [42])
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree