Intertrochanteric Hip Fractures
Extra-articular fractures of the proximal metaphyseal region of the hip, commonly referred to as intertrochanteric or pertrochanteric fractures, are the most common surgically treated adult long-bone injuries. They are of interest to the government, insurance companies, hospital administrators, surgeons, geriatricians, rehabilitation centers, and, most importantly, the patient, because they entail expensive treatments and vast time and resource dedication. Surprisingly, despite such attention, these fractures continue to have high mortality rates and outcomes that too often reflect permanent disability.
Brown et al1 provide estimates of the future incidence of these fractures in the United States and suggest two possible trends in frequency, yielding a conservative estimate of 458,000 fractures per year by 2050 and possibly as many as 1,037,000. The largest number of fractures will occur in women older than 65 years of age. In the Hip Fracture Database Report of over 59,000 cases from 180 hospitals in England, Wales, Northern Ireland, and the Channel Islands from 2011 to 2012, extremely detailed data have been obtained for the demographics of hip fractures. Interestingly, pertrochanteric fractures make up 34% of all hip fractures.2
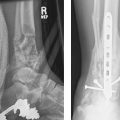
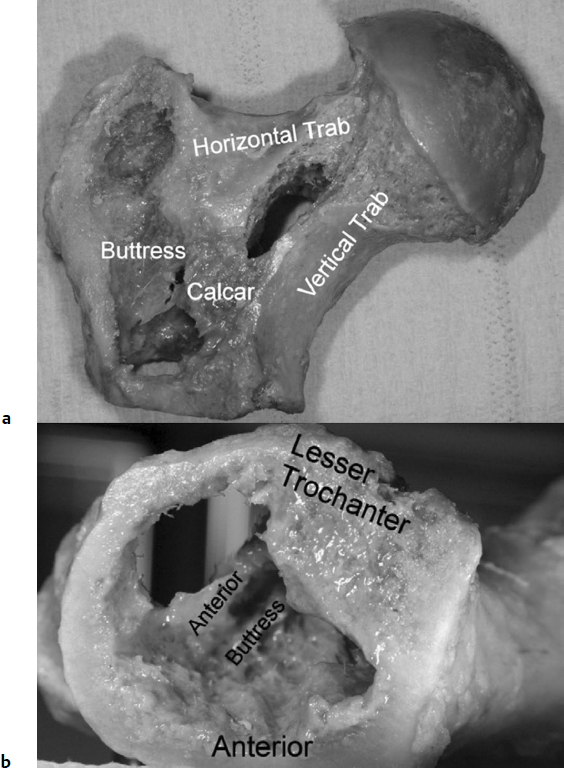
The anatomy of the pertrochanteric region of the femur is quite variable in its combination of cortical and cancellous bone structure. The well-vascularized pertrochanteric region is dependent on the structural integrity of a laminated cancellous bone arcade from the femoral head and epiphyseal scar, around Ward′s triangle, to the lesser trochanter, where the solid nature of the structure changes to a tubular construct with the origin of the femoral medullary canal; the strong plate of bone posteriorly is named the calcar femorale in the English-language literature, but was first described as “Adams’ arch” after Robert Adams in the mid-1800s.3 This is the region most affected in cases with posteromedial fracture comminution, which leaves only the anterior-medial cortex potentially stable for repair (Fig. 26.1).
The main structural attachments to the proximal femur include the hip capsule and the musculotendinous insertions of the gluteus medius and minimus (greater trochanter), iliopsoas (lesser trochanter), piriformis and short external rotators (posterior intertrochanteric ridge), the oblique head of the rectus femoris (anterior capsule), and the vastus lateralis (lateral femur just distal to the greater trochanter). The hip capsule is especially important in reduction of pertrochanteric fractures, and its continuity with the distal fragment is the soft tissue attachment on which a stable reduction is made possible.
With capsular disruption, the displacement of the fracture fragments is dependent on the musculotendinous attachment to the respective fragments. The greater trochanter is abducted and externally rotated by the gluteus medius and short external rotators, whereas the shaft is displaced posteriorly and medially by the adductors and hamstrings. This accounts for the usual shortening and coxa vara deformity of displaced fractures. With aging, the morphology of the hip changes with thinning of the cortex and expansion of the diameter of the bone. Younger hip fracture patients have a relatively narrow metaphysis, a high and narrow isthmus, and very thick cortical bone in the diaphysis. Further aging results in a slight widening and thinning of the cortex of the metaphysis, with bone loss and decreased thickness of the diaphyseal cortical bone stock and a widening of the isthmus. In the advanced age group, there is a very wide vacuous metaphysis proximally with loss of both tension and compression trabeculae, loss of the constriction of the isthmus, and a very round expanded tubular-shaped femur with a thin cortex.
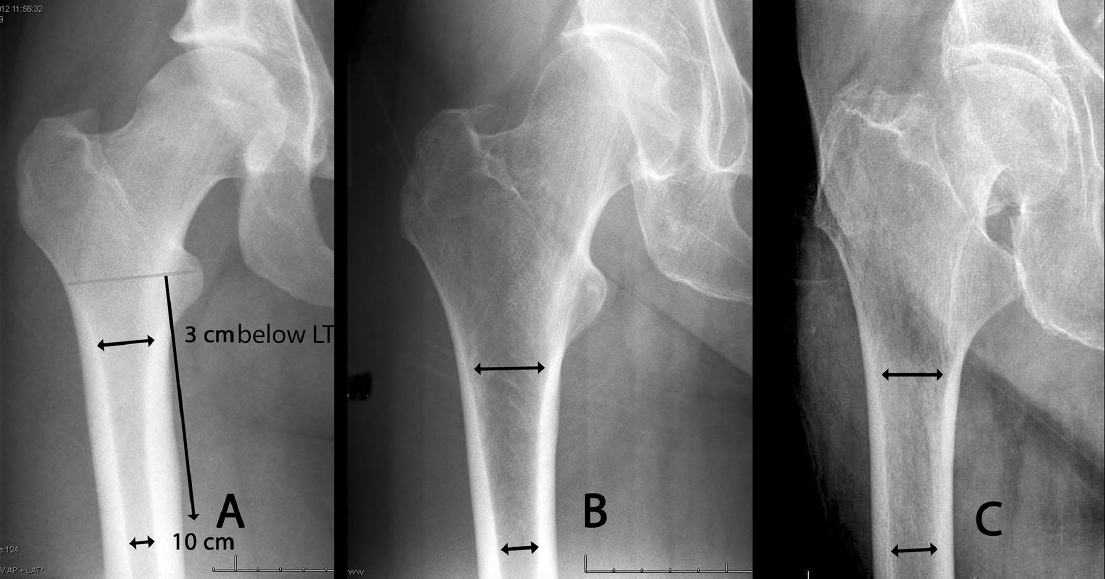
Three types of morphological anatomy were described by Dorr et al4 in 1993, referencing the selection of cemented versus noncemented femoral arthroplasty components (Fig. 26.2). The same rationale applies to implant selection for hip fracture patients. Type A, in young patients, entails a narrow metaphysis, thick cortex, and a high constricting isthmus. Excessive bone removal would be required for intramedullary devices, and either a plate-type construct or a smaller diameter reconstruction nail may be more bone conserving. Type B, in middle-aged patients, entails a wider metaphysis and a larger medullary canal, but relatively good cortex and isthmus constriction. Type C, in elderly patients, is the most problematic hip fracture, as it entrails a wide metaphysis, a wide medullary canal, and loss of the isthmus constriction, in association with loss of cortical diaphyseal and femoral head bone stock.
Patients most commonly present with a history of pain about the hip or knee and an inability to ambulate after a fall or other injury. The pain is localized to the proximal thigh and is exacerbated by passive or active attempts at hip flexion or rotation. The physical findings of a displaced hip fracture are shortening of the extremity and rotational deformity in the resting position compared with the contralateral extremity. Pain with axial load on the hip has a high correlation with occult fracture. The auscultation Lippmann test is quite sensitive for the detection of occult fractures of the proximal femur or pelvis.5 By placement of a stethoscope bell on symphysis pubis and tapping on the patella of both extremities, variations in sound conduction through the pelvis and hip from the patella result when there is any discontinuity. A decreased tone or pitch implies fracture within this arc of bone. Diagnosis is confirmed usually with radiographs, including an anteroposterior (AP) pelvis and AP and cross-table lateral of the affected hip. Fractures with subtrochanteric extension require full-length femoral AP and lateral radiographs for selection of implant length. If a long nail is being considered, then AP and lateral radiographs of the entire affected femur are required, paying special attention to the femoral bow and medullary canal diameter. Traction views in internal rotation may be of benefit preoperatively as an aide in the selection of definitive internal fixation (Fig. 26.3).6 In actual practice, the best radiographic analysis of the hip fracture occurs in the operative suite with fluoroscopic C-arm views, which provide immediate fracture analysis in complex fractures and feedback as to the stability of the fracture after the initial reduction. In many institutions this has led to elimination of preoperative lateral radiographs. Although this avoids subjecting the patient to a radiographic view that can be painful, the surgeon may not recognize displacement or fracture lines that might change implant selection that are only visible on the lateral image.

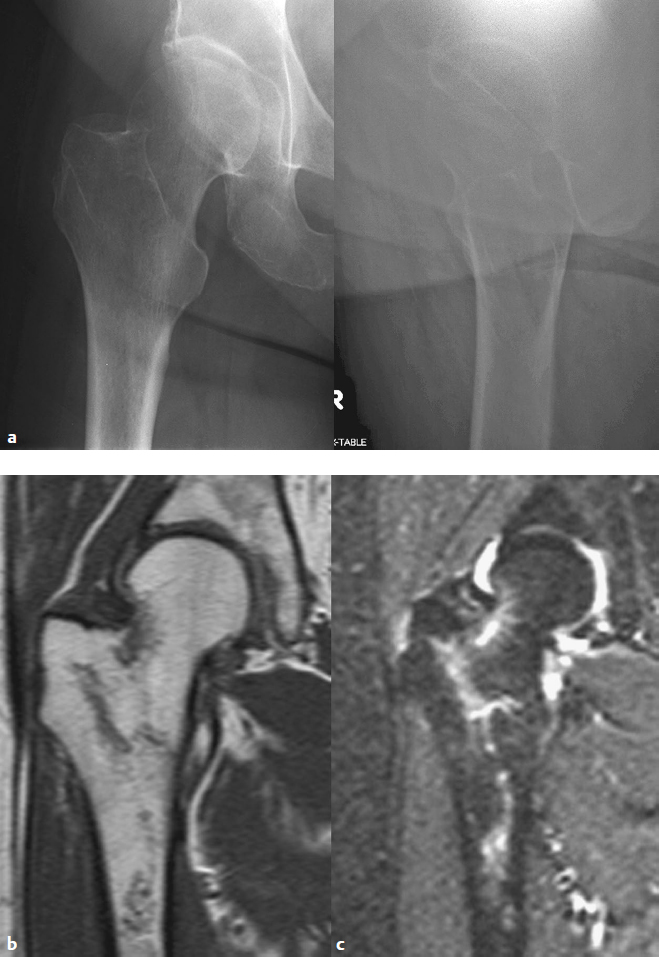
Computed tomography (CT) or magnetic resonance imaging (MRI) scans are rarely required for displaced fractures but may be useful in establishing the diagnosis in nonobvious or atypical fractures in high-energy trauma patients.7 MRI is preferred over CT or the older radionuclide scans due to its higher sensitivity and specificity, which facilitate a rapid decision process.8,9 If there is a clinical suspicion of hip fracture with normal radiographs, a T1-weighted coronal MRI is the best sequence of images for identifying a fracture (Fig. 26.4).10
Surgical research on internal fixation in the late 20th century focused on minimizing implant failure and avoiding cutout of the femoral head and neck fixation components, with the complicit acceptance of loss of reduction of the fracture. Functional recovery was not considered as being related to fracture malunion. Because many of these fractures are associated with osteoporosis, the current paradigm shift regarding hip fracture care relates to three main strategies: (1) prevention, through the aggressive screening and treatment of patients at high risk for fragility fracture; (2) standardization of hip fracture centers, with aggressive early intervention and protocols to avoid complications; and (3) optimization of the fracture reduction, and new designs of implant component fixation in osteoporotic bone with conceptual design changes in fixation stability and augmentation of the bone–implant interface.
Classification
The intent of the current classification systems of hip fractures is to identify those fractures that have high rates of implant failure, rather than to predict patient outcome. As such, the actual utility of presurgical classification is debatable. The common discriminators are radiographic findings, as opposed to physical examination; they include displaced versus nondisplaced, extension of the fracture zone to the proximal extracapsular region of the femoral neck versus extension below the lesser trochanter, and comminution and displacement of the medial or lateral walls of the pertrochanteric region. With a better appreciation of implant failure modes, adequate fracture reduction and iatrogenic damage during implant insertion have been reemphasized.11,12 Postsurgical evaluation of the fracture reduction and stabilization are more predictive of the outcome expected for each patient, as Evans13 originally reported. Conversely, many confounding psychosocial variables influence the outcome, including the patient′s preinjury ambulatory status, level of independence, and emotional well-being.
Over 30 classification systems have been published, with most noting similarities to the Evans classification. The AO/Orthopaedic Trauma Association (OTA) classification is now the most often cited classification in scientific articles. It is a derivative of the Müller classification (Fig. 26.5).14 The AO/OTA classification has nine main types; however, correlation is best when using only the three main categories. Also, there are no lateral radiographic parameters incorporated in the AO/OTA classification.15 Generally, fractures classified as 31A1 are the most stable, fractures classified as 32A2 are less stable, and fractures classified as 31A3 are the most unstable with plate device fixation. The AO/OTA classification has higher interobserver agreement than the Evans/Jensen classification, but neither meets the acceptable threshold for reliability.16,17
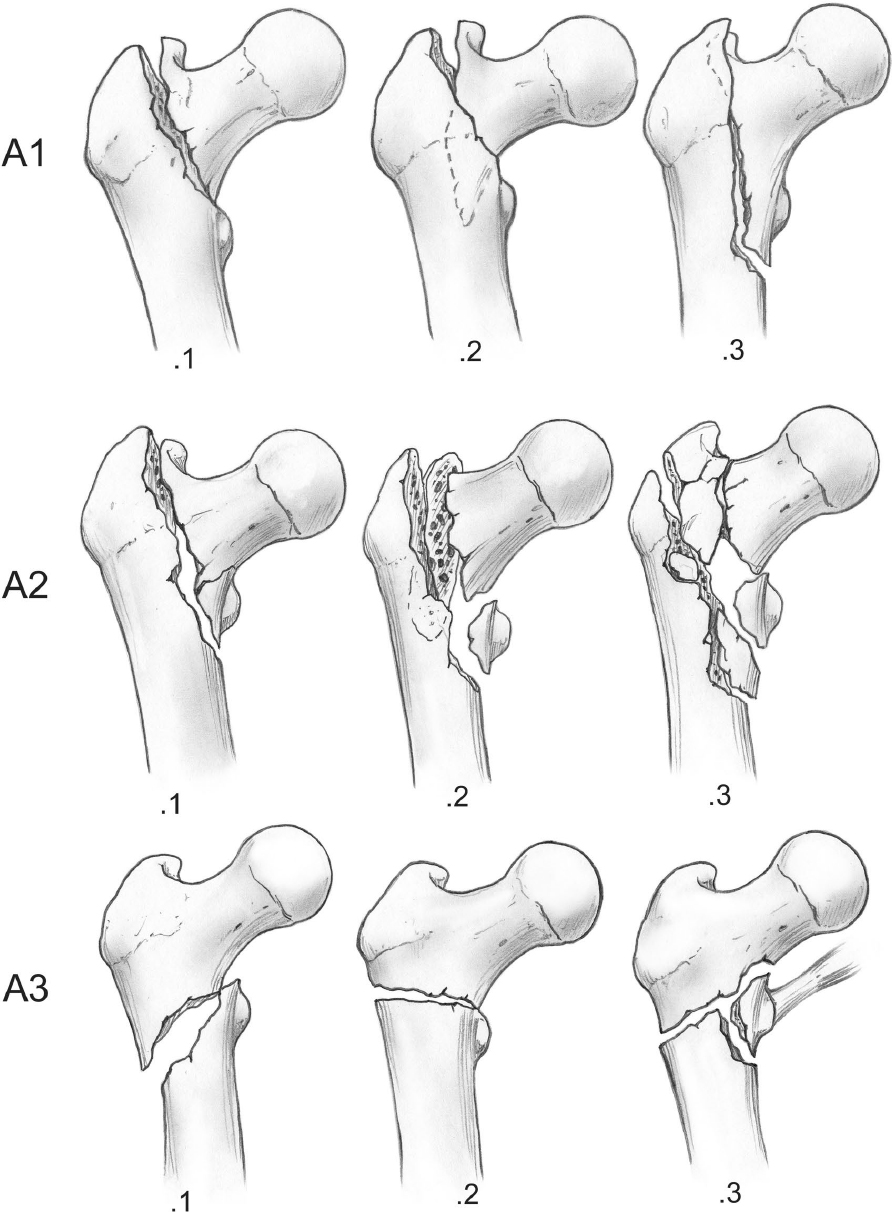
The OTA/AO classification is subject to periodic revision, and it is hoped that it will eventually incorporate in a reproducible manner new research on the issues of lesser trochanter comminution and lateral wall extension. Interestingly, in published reviews, reductions that these various classifications predict as being stable are not always stable. A 3 to 15% secondary displacement rate has been consistently reported after fixation of stable fractures.18 The rate of secondary displacement for unstable fractures is 11 to 67% in the literature. These findings raise doubt as to the utility of the classifications.
Nonoperative Treatment
Nonoperative treatment should be considered only in non-ambulatory or very low demand patients (such as those with severe dementia), whose pain is controllable with analgesics and rest, patients with terminal disease who have less than 6 weeks of remaining life expected, patients with unresolvable medical comorbidities that preclude surgical treatment, and patients with active infectious diseases that preclude insertion of a surgical implant. Exceptions include incomplete pertrochanteric fractures diagnosed by MRI, which have shown to heal with conservative measures in selected patients.19,20 If nonoperative care is selected due to an excessively high risk of mortality from anesthesia and surgery, if the patient is nonambulatory and has minimal discomfort from the fracture, or if modern medical facilities are unavailable, then rapid mobilization to a chair and an upright chest position is recommended. Mobilization is necessary to minimize decubiti, pneumonia, and dementia.
Bed rest with the lower extremity in extension and braced with pillows or pads for 1 to 2 weeks is usually required for pain control. Skeletal traction using either distal femoral or proximal tibial pins is usually necessary only for patients with subtrochanteric extension or preoperative flexion contractures of the hip. Nonoperative management also entails nursing care that includes attention to frequent alteration of positioning to avoid decubiti, attention to nutrition and fluid homeostasis, and adequate analgesics/narcotic pain suppression. Fracture callus formation at 3 weeks markedly decreases motion-related pain, and by 6 weeks most patients can be lifted into a wheelchair or reclining chair. Union occurs in 12 to 16 weeks.
Meta-analyses of randomized trials do not suggest major differences in outcome between conservative and operative management programs for extracapsular femoral fractures, but operative treatment appears to be associated with a reduced length of hospital stay and improved rehabilitation.21 Ambulatory capability after nonoperative treatment was traditionally viewed as poor, but some patients do regain some degree of ambulation ability.22,23
Surgical Treatment
Indications
Pertrochanteric fractures are globally viewed as an injury best treated with surgical repair. Surgeons must master multiple modalities of surgical treatment because the fracture patterns are not uniform, the morphology of the femur has significant variation, and the comorbidities of the elderly patient confound simple algorithms. Surgical management should be performed as soon as any correctable metabolic, hematologic, or organ system instability has been rectified, within the first 24 to 48 hours for most patients. The literature is suggestive of increased mortality after this time, but equally important is the increased patient suffering and hospital inefficiencies associated with unnecessary delay. Holt et al24 found that case-mix variables (age, sex, fracture type, prefracture residence, prefracture mobility, and American Society of Anesthesiologists [ASA] scores) were the critical predictors of mortality, even when corrected for the time interval between fracture and treatment, the time of admission, or the experience of the surgical and anesthetic staff undertaking the procedure.24 Centers with experience and protocols for the rapid diagnosis and treatment of hip fractures can effectively decrease the hospitalization time and complication risks for these injuries.25,26 Interestingly, earlier surgery has not been found to be associated with a higher mortality or morbidity,27 so the general dictum is to prioritize surgical treatment of hip fractures as urgent additions to the surgical schedule.
Implants
Surgical implant options included plate-and-screw constructs with either a nail or screws for femoral head fixation, intramedullary nail constructs with either a nail or screws for the femoral head, external fixation, and arthroplasty. Generically, these options can be grouped by design, with common biomechanical behaviors, techniques, complications, and results. The literature is exhaustive, with series of specific techniques and implants designs and a fair number of meta-analysis and randomized prospective studies, but controversy still abounds. New technologies are being developed to potentially augment the fixation of implants in the osteoporotic hip, thus addressing the main problem of bone interface failure.
Surviving the Night
Because many hip fractures present in the afternoon and early evening, by the time the patients have been evaluated and admitted to the hospital, the surgical team is fatigued, and only emergencies are considered for night surgery in most hospitals in the United States. Thus, there is a tendency to slow down the workup of the patient by anesthesia and internal medicine/geriatric consultants. But the patient must not be stranded for lack of a team leader. The optimal solution is a hip fracture team protocol that dictates the management of the patient, providing a framework to deal with presurgical stabilization and postoperative rehabilitation. As orthopaedic surgeons, we need to facilitate the preparation of the patient for surgery and recovery.
The best plan is to have a first-start initiative for hip fractures on the schedule. These patients tend to be older, dehydrated, and under-resuscitated after lying in pain and being kept on NPO status (nothing by mouth) all night. The entire exercise of preoperative preparation for surgery is an exercise in risk/benefit analysis. Hip fractures have a high potential mortality, and so the goal is to recover the patient to a pain-controlled, mobilizing, nutritionally restored status as soon as possible. Anemia, dehydration, and electrolyte abnormalities should be addressed on admission. Extensive cardiac workups that delay the surgery for days are an unnecessary hardship on the patient unless the patient is experiencing acute angina, an unstable arrhythmia, or severe valvular dysfunction on admission. Medical delay entails the detrimental effect of an increased risk of thromboembolic complications. Frequently, older patients are on anticoagulant therapy, but this should rarely preclude surgery within 24 to 48 hours.135
A standard protocol for hip fractures should be instituted, including protocols to deal with coagulopathy (international normalized ratio [INR] > 1.5), such as administration of vitamin K (1 mg IV) on admission.136 The application of traction to the injured limb has not been shown to offer any advantage over pillow support unless there is fracture instability extending into the shaft region. The administration of vitamin D (10,000 units) is recommended after drawing vitamin D levels. One must confirm correction of electrolyte anomalies, especially serum Na and K, as these are frequently correctable but delay treatment.
Parker and Handoll28 found that there is no consensus regarding the superiority of the dynamic compression nails or plate-type devices. Although the sliding hip screw (SHS) is still the most used device worldwide, it is associated with two serious complications: uncontrolled collapse and migration of the lag screw within the femoral head leading to varus malunion, and possible femoral head cutout. The latter is more likely to occur in malreduced fractures and those with iatrogenic fracture of the lateral wall, which leads to secondary instability of the fracture, construct collapse, and abductor insufficiency due to distortion of hip biomechanics. Gotfried has described this latter complication as a “pantrochanteric hip fracture,” caused by intraoperative, iatrogenic damage to the lateral wall of the proximal femur.29 The main advantages of the compression hip-screw implant and technique are that it is an inexpensive device and the technique of assembly is easily taught. In general, the cost of the implant can be a consideration, but not at the risk of compromising patient outcome. There is also evidence that some complications are more frequent with nail devices than with compression hip screws (CHSs). Despite this, the trend toward intramedullary fixation continues to increase in the United States.30 This trend may be related to surgeons’ familiarity with intramedullary nailing techniques and surgeons’ desire to avoid malunion and collapse.
Plate-and-Screw Fixation
Plate constructs may be grouped into four functional classes: (1) Impaction class. These constructs are impacted nail-type plate devices (e.g., blade plates, fixed-angle nail-plate devices). (2) Dynamic compression class. These constructs entail a large single sliding screw or nail for fixation of the femoral head with a side plate attachment (e.g., SHSs). (3) Linear compression class. These constructs are multiple femoral head fixation components controlling rotation and translation but enabling linear compression (e.g., Gotfried Percutaneous Compression Plating (PCCP; Orthofix, Lewisville, TX). (4) Hybrid locking class. These constructs are multiple fixation components with compression initially for fracture reduction, followed by locking screws that prevent further axial compression. These types of fixation are the most rigid (e.g., proximal femoral locking plates (Synthes, Paoli, PA; and Smith & Nephew, Memphis, TN). Lateral trochanteric buttress plates best fall into this category as well, because they rigidly stabilize the proximal femur (Fig. 26.6 and Table 26.1).

Impaction Class
Impaction or fixed-angle plating is most commonly used for corrective osteotomies rather than as a primary treatment for hip fractures. MacEachern and Heyse-Moore31 reported on the difference of failure mechanisms with medial penetration of the joint with the Jewett Nail compared with the SHS. Attempts at modifying the results of nail plates with osteotomies fell out of favor when comparisons were made to SHS devices, with anatomic reduction giving equivalent results with less blood loss and more rapid operative times.
In 1999, Chinoy and Parker32 performed a meta-analysis, comparing accurately fixed nail plates with sliding implants in 2,855 patients. Results showed an increased risk of cutout (13% versus 4%), nonunion (2% versus 0.5%), implant breakage (14% versus 0.7%) and reoperation (10% versus 4%) for fixed nail plates in comparison with the sliding implants. In addition, patients treated with fixed nail plates had a higher mortality, and the survivors were more likely to have residual pain in the hip and impaired mobility.
Dynamic Compression Class
Video 26.1 ORIF Using Sliding Screw and Side Plate
From the 1980s to 2000, sliding CHSs became the gold standard for hip fracture fixation, and many surgeons still advocate their use in all fractures, largely due to the reports of Clawson33 and Mulholland and Gunn,34 and meta-analyses by Parker and Handoll35 from 2000 to the present. The device consists of a large fragment side plate attached with 4.5-mm cortical screws to the shaft of the femur, with a barrel on the proximal plate for connection with a large threaded screw inserted over a guidewire into the femoral head. These devices, made from stainless steel or titanium alloys, come in varying barrel angles of 125 to 150 degrees, with large-diameter lag screws of about 12.5 mm, in lengths ranging from 65 to 130 mm. The plates typically have two to four holes, and longer versions are available. The plates are commercially available worldwide as generic devices from many manufacturers.
Patient Positioning
The patient is moved to the fracture table after anesthesia is completed to minimize pain. A supine position, with bilateral foot traction and with the knees in extension and the legs scissored, is optimal. The operative leg is raised to approximately 20 to 30 degrees of flexion, and the non-operative extremity is extended 20 to 30 degrees. The legs are pulled in-line with the body to avoid varus positioning of the hip. The C-arm is brought in from the opposite side with the base parallel to the operative extremity centered on the mid-femur such that the cephalad-caudad movement of the C-arm enables good visualization of the femoral head and shaft in AP and lateral views. With this type of setup, the true AP view of the hip is usually obtained with 10 to 20 degrees of rotation of the C-arm over the top, and the true lateral view corresponds to approximately 15 to 30 degrees over the horizontal position (Fig. 26.7). In obese patients, the option of flexing the nonoperative hip and bringing the C-arm between the legs may provide better visualization of the hip, but one must be aware of the potential for compartment syndrome development in the flexed uninjured limb with prolonged elevation.

My preferred technique for reduction of the proximal femur fracture involves a four-step technique (Table 26.2):
After attachment to the foot positioner with the perineal post attached, correct the posterior sag at the fracture with a posterior to anterior directed force and maintain it.
Flex the leg through the foot holder 20 to 30 degrees from neutral for intertrochanteric fractures and 30 to 40 degrees for subtrochanteric fractures, while maintaining the posterior to anterior reduction force at the hip, centered on the shaft fragment.
To restore length, apply traction in line with the body. Do not accept any varus malreduction. Traction should not be too powerful, as it may disrupt any remaining soft tissue attachments and further destabilize the fracture.
Rotate the leg to align with the proximal fragment, 5 to 15 degrees of external rotation for most subtrochanteric fractures and 0 to 10 degrees of internal rotation for pertrochanteric fracture, verified with image verification.
Surgical Approach
The lateral approach to the proximal femur for plating has been relatively standardized over the past 70 years. The surgery is commonly performed on a fracture table, with the patient′s leg and foot secured after closed reduction. The entire proximal thigh from the iliac area to the distal femur is prepared in the standard fashion. The incision length is based on the length of the proposed plate-shaft component; the incision is started with reference to the intraoperative C-arm fluoroscopy views and is centered over the lesser trochanter. Commonly the incision length is 5 to 10 cm. The iliotibial band is incised longitudinally, and the proximal portion is extended sufficiently to develop the area of the intertrochanteric line for palpation anteriorly, usually just proximal to the origin of the vastus lateralis. The fascia of the vastus lateralis is incised near its attachment posteriorly at the linea aspera. Leave sufficient fascia posteriorly (5–10 mm) for closure and to identify and obtain hemostasis of all perforator arteriovenous structures. Reflect the vastus anteriorly, exposing the lateral femoral shaft; detachment of the origin of the vastus may be required for fracture visualization and reduction. There are no significant neurologic or vascular structures at risk with this approach (Fig. 26.8).
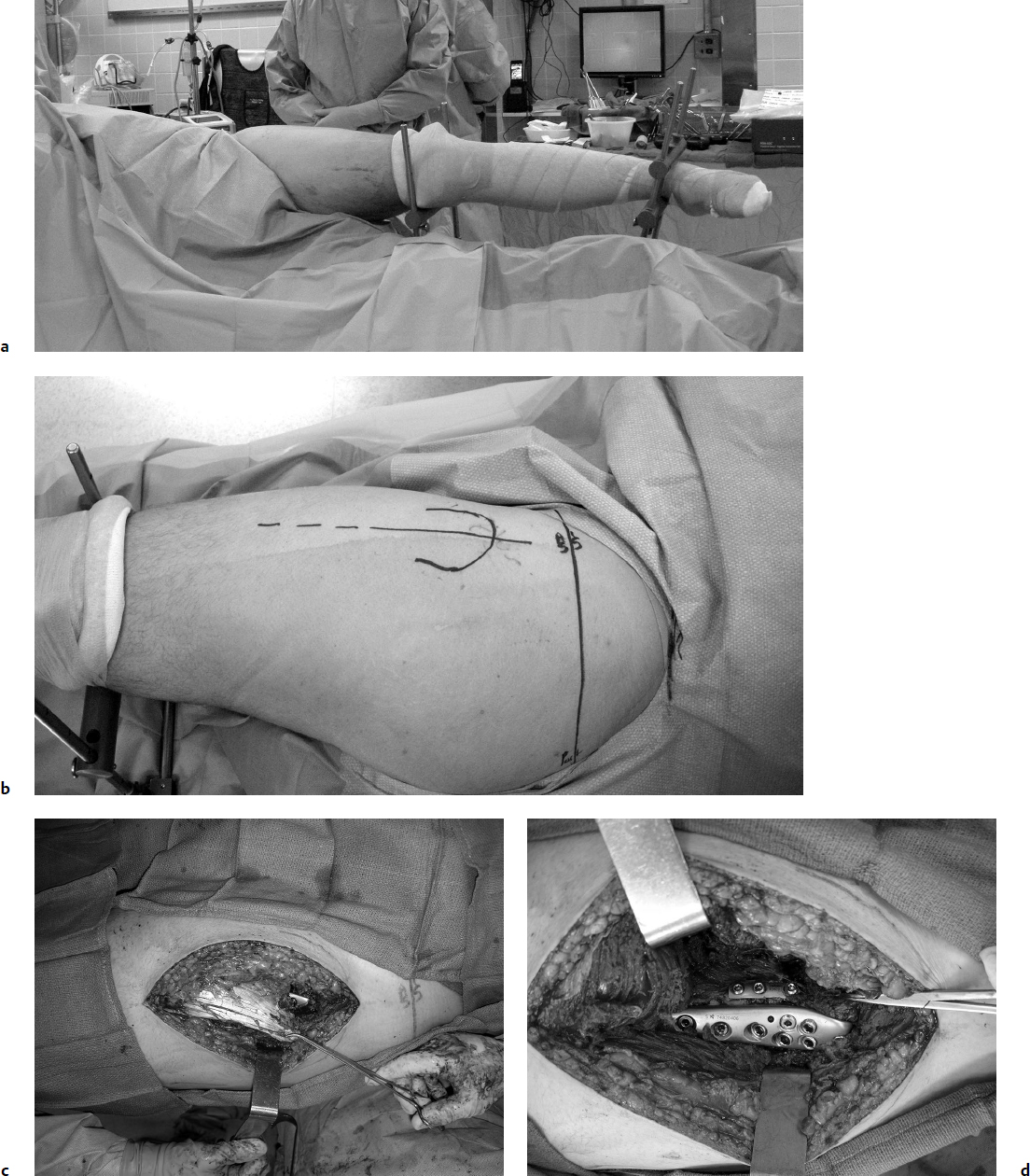
For more extensive dissection, the Watson-Jones anterolateral approach to the hip is used. It is a proximal expansion of the straight lateral approach previously described. The muscular interval proximally is between the tensor fasciae latae and the gluteus medius. The interval between these two muscles is best begun distally and exposed proximally. Follow the anterior border of the vastus lateralis proximally to reach the anterior trochanteric ridge and hip capsule. The use of Schanz pins drilled into the proximal femur is an aid in retraction for better visualization and may be used for manipulation of the shaft (Fig. 26.9). For pertrochanteric fracture management, further capsulotomy and greater trochanteric osteotomy are rarely required. The main vascular obstacle is the ascending branch of the lateral femoral circumflex artery, which should be isolated and ligated in the approach. Complete proximal dissection of the gluteus medius and tensor fasciae lata interval to the iliac crest is rarely necessary; the superior gluteal nerve to the tensor fasciae latae will be sacrificed with full proximal dissection, but this is not clinically significant.

Surgical Technique
Anatomic reduction is often difficult, but an emphasis on the anterior medial cortex reduction will most often be the key to success. One should not assume that any implant would substitute for a lack of reduction. In 1963, Sarmiento36 called attention to the key components of reduction of the pertrochanteric fracture:
Weight-bearing on the fractured extremity is safe only if the fracture, whether simple or comminuted, has been reduced so that there is an accurate fit of the fragments at the anteromedial cortex of the femur. Absorption of the reduced medial cortex of the femur with loss of stability is unlikely because of the great thickness and strength of the anteromedial cortex. Failure to obtain such reduction because of the degree of comminution or technical difficulties precludes weight-bearing until bone union is complete. Anatomical reduction of the medial and anterior cortices is of great importance since the stability of the fracture and the efficiency of the nail depend on the reduction of this portion of the bone.
It is commonly assumed that internal rotation is the correct position for the hip fracture reduction, but according to studies by Bannister et al37 and May and Chacha,38 pertrochanteric fractures may have an equal chance of being reduced in internal or external rotation. Excessive internal rotation can lead to posterior gapping, further destabilizing the fracture with a large posteromedial defect. Ramanoudjame et al39 recently reported a 40% malrotation rate of over 15 degrees among intertrochanteric fractures treated with CHSs or nails when reduction was evaluated with CT scan.
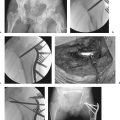
Carr12 renewed Sarmiento′s36 focus on the anteromedial cortex and described reduction techniques to facilitate the reduction. Reduction is assessed by palpation of the region of the intertrochanteric fracture line anteriorly. The surgeon should search for a step-off, which identifies where the posteriorly displaced shaft is overlapped by the anteriorly displaced head and neck fragment. First, pull the shaft laterally to disimpact it from the head and neck fragment. This can be done with a bone hook passed around the femoral shaft in a subperiosteal manner distal to the lesser trochanter. With the shaft retracted laterally, insert a narrow Jocher or Key elevator between the head and neck shaft pieces, and lever the head and neck piece anteriorly, while applying a posterior force to the shaft to align the two fragments (Fig. 26.10). Adjustments in the length or rotation of the limb may be required at this time. Release the laterally directed traction on the shaft. Confirm the anterior reduction with C-arm views.
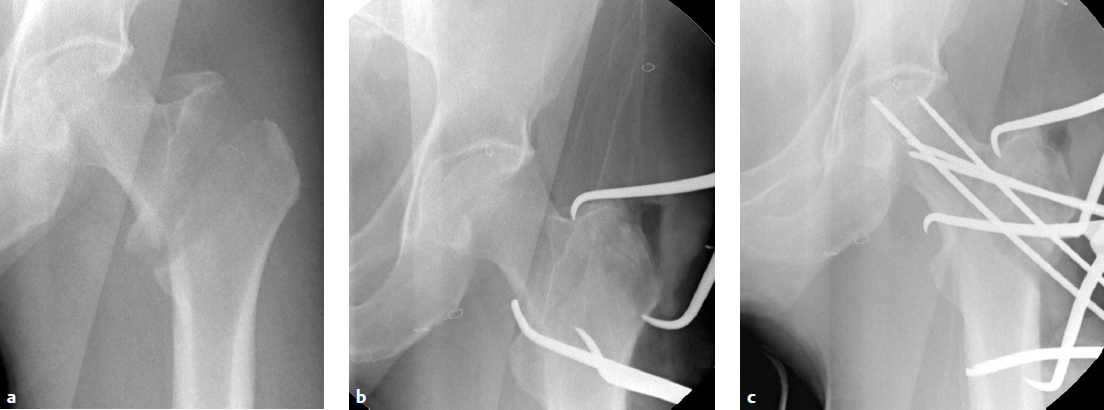
At this point, AP radiographic views of the hip reveal an anatomic neck shaft junction that is manifested as a hair-line crack reduction. On the lateral view, the anterior cortex line is reestablished. Secure the reduction with one or two 3.2-mm wires that are directed away from the area of intended lag-screw placement. Be careful that the anterior reduction is not lost during insertion of the hip compression screw, which tends to rotate the head and neck fragment. The lack of adequate provisional fixation is the most common reason that obtained reductions are lost during fixation. Provisional fixation must be placed so that it will not interfere with the definitive fixation. Alternatively, fixation can be achieved in long oblique fractures by Kirschner wire (K-wire) fixation from the anterior longitudinal shaft region into the medial femoral neck (Fig. 26.11a,b). Weber clamps with a wide jaw may be inserted in a limited open reduction in conjunction with a bone hook along the medial cortex or medial to the greater trochanter to achieve fracture reduction. If there is posterior displacement of the distal fragment, there are no soft tissue structures to leverage, and a manual reduction of the shaft to an anterior position will be required. These displacements require a combination of traction, translation of the distal fragment, and some degree of rotational correction. Rotational reduction is best evaluated by intraoperative radiographic views.

After provisional fixation in an anatomic reduction is secured, the position for the plate is determined. Care must be taken such that the plate is placed in line with the correct trajectory into the femoral head but also aligned with the shaft. The tendency is to place the plate too anteriorly. A provisional 3.2-mm K-wire can be introduced over the anterior femoral shaft under image control, centered over the femoral neck, and tapped into the femoral head to provide guidance for the projected plate position. This K-wire penetrates the capsule of the head and is safe as long as it is kept on the anterior surface of the bone. The orientation of this wire in the sagittal plane indicates the anteversion of the proximal femur and also the relative neck-shaft angle, as originally described by Tronzo.40
Next, a 3.2-mm guide-pin is introduced into the femoral head using an appropriate 125- to 135-degree angle guide. This pin is introduced at the level at the lesser trochanter and parallel with the previous anteversion pin. In hard bone, it may be helpful to pre-drill with a 4.5-mm drill bit to facilitate guidance of the guidewire into the center of the femoral head. The guidewire is then inserted, centered on the AP and lateral views in the femoral neck. It is important that the wire should not be positioned too anteriorly or too posteriorly from its entry portal and should be centered on the AP and lateral X-rays into the femoral head. An important concept to understand regarding ideal position of the guide pin in the femoral head is the tip–apex distance (TAD) described by Baumgaertner et al.41 The TAD is a measure of the distance from the apex of the femoral head to the tip of the inserted guide pin as measured on both the AP and lateral radiographic views. The TAD is the sum, in millimeters, of the distance on the AP view from the tip of the implant added to that on the lateral view (Fig. 26.11c). The risk of cutout increases dramatically when the TAD exceeds 25 mm. The guide pin should be advanced to within 5 to 10 mm of subchondral bone to meet the requirements of the TAD, as described by Baumgaertner et al, of less than 25 mm.
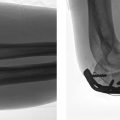
Next, the length of the guide pin is measured, and usually a triple-reamer type device is then used to prepare the bone for insertion of the lag screw into the femoral head and to prepare the lateral cortex of the femur for the barrel of the side plate. This plate is reamed to the depth indicated by the guide pin. Usually a screw is selected that is 5 mm shorter than the guide pin. If the bone is dense, it should be tapped, and provisional fixation should be maintained to the fragment before tapping to ensure that there is no loss of rotation due to the rotation of the tap. Once the screw has been selected, it is inserted, usually with a cannulated attachment over the guidewire and seated to within 5 to 10 mm of subchondral bone. The selected side plate usually has two to four holes: two holes for simple two-part stable fractures, and longer plates for more unstable patterns or in patients with osteoporotic bone. The plate is applied and inserted. If the plate does not readily sit flush on the bone, the wrong plate-barrel angle may have been selected, and care should be taken not to force the reduction of the plate to the bone because this may result in a gapping of the medial cortex with induced deformation of the reduction. The sliding mechanism will not compensate for a distracted anteromedial reduction and will result in premature plate failure by pull-off from the shaft or cutout from the femoral head (Fig. 26.12).

After the side plate is applied and clamped to the femoral shaft, a lag screw is applied with a standard drill bit (3.2- or 3.5-mm depending on the system), and a 4.5-mm cortical screw is inserted in the proximal cortex position. One should determine that the plate is longitudinally aligned with the shaft of the femur to avoid offset or eccentric, unicortical screws distally. Next, release the traction and impact the plate with an impactor to ensure lateral plate contact with the shaft; watch out for plate malrotation that can cause the plate to flex or extend relative to the femur so that the distal screws become unicortical. The importance of provisional fixation during screw insertion was discussed by Mohan et al,42 who showed that malreduction occurred with tightening of the lag screw in left-sided patients with malreduction. Most systems have compression screws that can be inserted through the plate into the large lag screw so that compression can be applied after traction is released. Excessive compression should be avoided, as it may disrupt the fixation of the head and render the construct unstable. Also, in anticipation of up to 5 mm of sliding, the screw should not be brought out so far that it protrudes past the plate, as this may cause pain from the implant postoperatively. Excessive femoral neck shortening is a problem that is not addressed with the SHS even with optimal reductions (Fig. 26.13). Whether the compression screw device is left in place is up to the surgeon; there are no clear data as to the benefits of compression screw removal or retention. The plate is then further secured with 4.5-mm screws with bicortical fixation. Hemostasis is confirmed and the wound is closed in layers in the standard fashion. Drains are not routinely used unless the patient is on anticoagulant therapy. Intraoperative confirmation of the reduction on the AP and lateral views and a radiographic record are obtained. Critical factors that affect the stability of fracture reduction are summarized in Table 26.3 .

Postoperative Care
Anteroposterior and lateral radiographs of the final construct should be obtained in the surgical suite to assess the construct and ensure its stability. If there are adjustments to be made, they are best made while the patient is still under anesthesia. Radiographs should depict the entire fracture region, including the entire implant construct. Patients are mobilized to a chair upright position the day following the operative procedure. Ambulation is begun under supervision, with weight bearing as tolerated and a walker or crutches, with emphasis on heel-strike and upright balance exercises. Ambulation is delayed in patients with multiple trauma or other complications, but it should begin as soon as possible to minimize secondary complications. In patients with hip fracture, delay in mobilizing the patient is associated with poor function at 2 months and worsened 6-month survival.43 Studies also reveal decreased rates of pneumonia, urinary tract infection, and dementia with early mobilization.44 Besides accelerating recovery, the ability to ambulate postoperatively is prognostic for survival rates.45 Specific fracture service and rehabilitation protocols reduce the 30-day mortality rate from 22% to 7%.46
Weight bearing is most important in these patients for optimal recovery and to assuage the fear of falling and lack of independence. Weight-bearing stability of the implant construct improves the functional ability of the patients.47 There must be sufficient stability to allow aggressive physical therapy, especially in the cognitively impaired patient with hip fracture.48 Koval et al49 reported that patients will autoregulate their weight bearing.
Postoperative pain control is also important for recovery. Patients with higher pain scores at rest had significantly longer hospital stays, were more likely to miss physical therapy sessions or leave them early, were less likely to be ambulating by postoperative day 3, took longer to ambulate past a bedside chair, and had significantly lower locomotion scores at 6 months.50 There is an important relationship between adequate pain control and implant stability. If an implant is unstable in the first 6 weeks after surgery, pain and lack of mobility may affect long-term functional recovery.
Protein and caloric nutrition and osteoporotic therapy including vitamin D supplementation are important for successful recovery.51 Bilateral hip abduction exercises in conjunction with proper balance and gait training are required; crutches or a walker should not be abandoned until normal gait is restored. Patients must be counseled to report any increased swelling or respiratory distress as an emergency due to the high risk of thromboembolic disease. On discharge, patients are prescribed vitamin D (minimum of 1,000 IU daily); if the patient′s vitamin D level is low, consider prescribing 50,000 IU weekly for 12 weeks. Fall-prevention education and safe-home checks should be provided to the patient′s family or social support group. Patients are reevaluated with exam and radiographs at 2 weeks and then monthly thereafter until fracture healing is documented and the patients have maximized their ambulatory capabilities, usually by 6 months after injury.
The American Orthopaedic Association (AOA) has recommended that orthopaedists include a plan to identify secondary causes of osteoporosis, obtain bone density testing, and initiate osteoporosis pharmacotherapy if appropriate. The Fracture Risk Assessment Tool (FRAX) calculator (http//www.shef.ac.uk/FRAX/) is a free service for patients not on osteoporosis treatment and is helpful for patient education and risk assessment.52 The American Academy of Orthopaedic Surgeons (AAOS), the AOA, and the Osteoporosis Foundation encourage the assessment of fragility fracture and osteoporosis for diagnosis in the discharge summary. Bisphosphonate therapy should begin within 2 weeks after surgery. The major risk factors for geriatric fractures include prior fragility fracture, increased age, low bone mineral density, low body weight, family history of osteoporotic fracture, glucocorticoid use, secondary osteoporosis, liver and kidney disease, Crohn′s disease, rheumatoid arthritis, hyperthyroid disease, antiepileptic medications, primary hyperparathyroidism, systemic inflammatory disease, smoking, and vitamin D deficiency.53,54
Boonen et al55 reported that the drugs alendronate and risedronate are effective in decreasing the risk of hip fractures. The AOA′s “Own the Bone” initiative notes that patients with a hip fracture have at least one treatable secondary cause of osteoporosis, usually low vitamin D (osteomalacia).56 Similarly, 40% of women and more than 60% of men with osteoporosis have a secondary condition and a treatable cause of their low bone density (Table 26.4).
The “Own the Bone” initiative recommends the following:
Prescribe calcium (1,200 mg daily).
Prescribe vitamin D (minimum of 1,000 IU daily; if the patient′s vitamin D level is low, consider prescribing 50,000 IU weekly for 12 weeks).
Refer the patient to physical medicine or physical therapy for fall-prevention education.
Ask the family or friends to perform a home-safety check.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree