This review explores the clinical presentation of lower extremity DVT and pulmonary embolism (PE), treatment strategies, and outcomes for venous thromboembolism (VTE) in the pediatric population. Traditional therapy for pediatric VTE was anticoagulation alone with thrombolysis and surgery reserved only in life or limb-threatening cases. Catheter-directed thrombolysis (CDT), pharmacomechanical thrombectomy (PMT) and mechanical thrombectomy (MT) have emerged as effective and safe treatment options for VTE management. Although most data are from adult studies, early pediatric studies suggest that these interventional procedures can be effective in children. The significant clinical impact of post-thrombotic syndrome (PTS) is also discussed, as PTS can lead to lifelong physical symptoms and psychosocial damage.
Deep vein thrombosis
History and physical
Following the objective diagnosis of lower extremity DVT in pediatric patients, the initial evaluation is like that of adults and should begin with the patient and family history to identify VTE risk factors. Additionally, a review of known compression syndromes such as May-Thurner (left iliac vein compression by crossing right iliac artery) and variations in anatomy such as inferior vena cava (IVC) agenesis/atresia will help include anatomical predisposing factors for lower extremity DVT.
Precipitating factors and the duration of symptoms may determine the chronicity of the thrombus. Symptom duration determines the chronicity of a thrombus: less than 14 days (acute), 15-28 days (subacute), and greater than 28 days (chronic).
A physical exam should include an assessment of pain, edema, coloration, pulse, strength, and sensation. Symptoms of iliocaval DVT are variable and can include back pain, pelvic pain, thigh pain, and exercise intolerance. The presence of collateral circulation is an indicator of subacute or chronic disease and can be visible on exam. Venous stasis, skin changes, and ulceration suggest chronic DVT.
Laboratory
In collaboration with our hematologists, our institution has developed a multidisciplinary care pathway. Hematologists guide the laboratory evaluation. For example, in a child with unprovoked DVT, it may be helpful to test for coagulopathies such as deficiencies of protein C, protein S and antithrombin, the factor V Leiden and prothrombin mutations, antiphospholipid antibodies, elevated factor VIII, and lipoprotein (a), and rarely homocysteine levels. Alternatively, in the setting of severe, progressive DVT or perceived thrombotic storm, erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP) can help guide the use of steroids, rituximab, or intravenous immunoglobulin (IVIG); these therapies calm the damaged venous endothelium.
Imaging
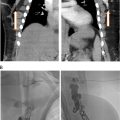
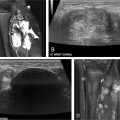
Determining the proximal and distal extent of the lower extremity DVT is critical and best-done using ultrasound, which has a sensitivity of 96% and a specificity of 96% above the knee. The ultrasound technique for DVT evaluation should include compression, color Doppler, and spectral Doppler. Particular attention should be paid to the waveform of the common femoral vein, as dampening may indicate proximal iliac vein or IVC thrombus.
In our practice, we image the iliac veins and inferior vena cava using magnetic resonance imaging (MRI) or computed tomography (CT) with scanning timed to the venous phase. MR venography with contrast is the preferred imaging modality as there is no ionizing radiation, venous timing in the pediatric population is better achieved, and congenital and pathological abnormalities are well delineated. MR venography requires longer exam time which may require sedation. CT venography is rapidly acquired and likely not to require sedation but involves ionizing radiation, and optimal contrast timing can be challenging in pediatric patients.
Indications for the procedure
Goal of therapy
Many pediatric hospitals follow the 2012 American College of Chest Physicians and the 2018 American Society of Hematology guidelines, which recommend anticoagulation as the primary treatment for pediatric DVT, with endovascular intervention reserved for severe, life- or limb-threatening cases. ,
However, there is a trend towards intervention for occlusive DVT extending into the iliac vessels or IVC to prevent PTS, a condition of chronic venous insufficiency causing pain, edema, poor wound healing and potentially severe skin issues (dermatitis, venous ulcers). PTS can have devastating impact on children with numerous physical and psychosocial downstream effects. Preventing PTS may not only improve the quality of life for the patient but also have an economic impact given the cost of debilitating PTS.
Additionally, interventions aim to prevent thrombus extension, pulmonary embolism, recurrent thrombosis, and loss of central venous access in patients with chronic conditions requiring long-term venous access, such as cystic fibrosis, congenital heart defects, sickle cell anemia, short gut syndrome, and post-transplant cases. At our institution, we have extrapolated the ATTRACT trial results to pediatrics, focusing on interventions that include a combination of anticoagulation with CDT, PMT, or MT for iliofemoral thrombus with the goal of prevention or reduction of the severity of PTS.
Choosing the appropriate therapy
Anticoagulation is the initial therapy for DVT and is initiated immediately after diagnosis. Options include unfractionated heparin, low molecular weight heparin, direct thrombin inhibitors such as bivalirudin, or increasingly direct oral anticoagulants (DOAC) for outpatient treatment. Anticoagulation prevents progression of thrombus and relies on intrinsic thrombolytic mechanisms for thrombus dissolution. Although effective and safe, the process can be slow or incomplete. Protracted course may result in prolonged symptoms, valvular damage, collateral vessel formation, venous hypertension, and PTS. Thrombolytics like recombinant tissue-type plasminogen activator (tPA) dissolve acute thrombus but have increased risk of major bleeding. Absolute contraindications include active bleeding, a cerebrovascular event in the last 3 months, and other contraindications to anticoagulation. Relative contraindications include recent surgery, right-to-left shunt, recent solid organ biopsy, recent GI hemorrhage, uncontrolled hypertension, hemodynamic instability, severe thrombocytopenia, and infection.
The risk of bleeding influences the choice of intervention, with mechanical thrombectomy being preferable in high-risk cases. The “open vein hypothesis” has led to the implementation of both CDT and PMT for acute DVT. , Our institution’s clinical care pathway includes the early involvement of a pediatric hematologist to help facilitate appropriate consultations and discussions regarding intervention. Emergent endovascular intervention for DVT is usually unnecessary except in severe cases such as free-floating IVC thrombus or phlegmasia cerulea dolens resulting in compressive arterial insufficiency and eventual ischemia.
Equipment
Aside from equipment for standard vascular access and balloon angioplasty, special devices are needed for endovascular intervention. See Table 1 and Figures 1 and 2 for our institutional practices.
| Procedure Type | Device | Indications |
|---|---|---|
| Catheter directed thrombolysis (CDT) | Uni-Fuse (Angiodynamics, Latham, NY) |
|
| Cragg-McNamara (Medtronic, Dublin, Ireland) |
| |
| EKOS endovascular system (Boston Scientific, Marlborough, MA) |
| |
| Pharmacomechnical Thrombolysis (PMT) | Angiojet (Boston Scientific; Marlborough, MA) |
|
| Bashir catheter (Thrombolex, New Britain, PA). |
| |
| Deep vein thrombus | ||
| Mechanical Thrombectomy (MT) | Indigo Aspiration System, Lightning (Penumbra, Inc; Alameda, CA) |
|
| ClotTriever (Inari Medical; Irvine, CA) |
| |
| ||
| FlowTriever (Inari Medical, Irvine, CA) |
| |
| Indigo Aspiration System, Lightning (Penumbra, Inc; Alameda, CA) |
| |
| AngioVac (Angiodynamics, Latham, NY) |
| |
| AlphaVac (Angiodynamics, Latham, NY) |
| |
| Additional pertinent equipment | ||
| Intravascular Ultrasound (IVUS) | Philips; San Diego, CA |
|
| High Pressure Balloons | Multiple brands |
|
| Variety of stents | WALLSTENT (Boston Scientific, Marlborough, MA) Abre (Medtronic, Minneapolis, MN) Venovo (BD Bard, Franklin Lakes, NJ) |
|