KEY POINTS
One of the most common and usually benign fetal brain tetralogy pathology.
Easily detected due to its obvious anechoic appearance at places that should not be located present.
Larger cysts may exert pressure on adjacent organs causing displacement of structures or obstruct the flow of cerebrospinal fluid.
Although mostly isolated, they can be associated with other pathologies, therefore targeted neuroscan and anatomy scan is warranted.
Intracranial cysts are relatively common findings encountered in the prenatal sonographic assessment of the fetal brain. The vast majority of these fluid collections (arachnoid and choroid plexus cysts) are of a benign nature, remain clinically silent, do not evolve, and regress spontaneously. When these lesions are not associated with other fetal anomalies, they are compatible with normal life regardless of whether they require postnatal treatment or not.1,2 Nevertheless, due to the numerous differential diagnostic entities, as well as the associated parental anxiety, clinically, these findings present a dilemma in need of appropriate diagnosis and counseling.
The differential diagnoses of intracranial cysts encompass multiple etiologic and pathologic processes. Advancements in imaging techniques, especially in fetal sonography, have facilitated the workup of such cysts by depicting their exact location, size, relationship to the ventricular system, and midline structures. Additionally, fetal sonography allows for evaluation of the presence of solid components seen in cases of brain tumors or blood clots and for performance of Doppler studies of blood flow patterns in cases of vascular malformations, such as an aneurysm of the vein of Galen. Nevertheless, imaging studies may come short of distinguishing between some of these lesions, which require histologic examination of the cyst wall to establish the correct diagnosis.3 In this chapter, we classify intracranial cysts based on the location of their origin into one of three groups: extra-axial, intraventricular, and intraparenchymal (Table 11–1).
| Extra-axial Cyst | Intraventricular Cyst | Intraparenchymal Cyst |
|---|---|---|
| Arachnoid cyst | Choroid plexus cyst | Periventricular pseudocyst |
| Glioependymal cyst | Choroid plexus hemorrhage | Cystic periventricular leukomalacia |
| Endodermal cyst | Porencephalic cyst | |
| Dural separation | Cystic brain tumor | |
| Cystic tumors (teratoma) | Holoprosencephaly | |
| Vascular malformations | Schizencephaly |
This group of lesions consists of arachnoid cysts, glioependymal cysts, endodermal cysts, cystic teratomas, and dural separation due to dural sinus thrombosis.
Arachnoid cysts are by far the most common lesion in this group, and our review will focus on them.
None
Arachnoid cysts were first described by Bright in 18313 as “serous cysts forming in connection with the arachnoid and apparently lying between its layers.” Like other intracranial cysts, arachnoid cysts are collections of cerebrospinal fluid (CSF) on the brain surface bordered by a cyst wall.
Arachnoid cysts account for 1% of all intracranial masses in children.2,3,4 They usually occur in a sporadic fashion as an isolated single lesion. Multiple and bilateral arachnoid cysts are unusual; familial occurrence has been reported in only a few cases. The left side of the brain is affected more commonly, and a male predominance with a 2:1 male-to-female ratio has been shown.5,6,7,8
The exact pathogenesis is not clear, but it is thought to develop mostly in the second and third trimesters. Arachnoid cysts are usually benign, congenital, space-occupying lesions with a cavity that is entirely surrounded by a transparent arachnoid membrane. These collections of CSF are located within the layers of the arachnoid membranes and may or may not communicate with the subarachnoid space. Postnatally, they usually are the result of head trauma, which provokes a proliferation of fibroblasts that form a loculated cyst within the leptomeninges. These can enlarge because the fluid within (CSF) is trapped and can only increase. As a congenital lesion, the cause is less certain, but the prevailing theory is that an arachnoid cyst is a disturbance of the mesencephalic neural crest, the origin of the meninges, which forms a splitting of the primordial membrane into which fluid accumulates and becomes loculated, analogous to a dissecting aneurysm. Whereas a cleft may form during this period, it does not necessarily fill with fluid to separate the two leaves until later in gestation or even postnatally; hence, it is plausible that it would not be identified in the second trimester by neuroimaging, because it may be detectable only microscopically at that time.
An arachnoid cyst may present as a primary or an acquired lesion. Primary cysts commonly arise from an abnormal developmental process of the leptomeningeal formation, whereas acquired arachnoid cysts (secondary cysts) generally result from entrapment of CSF within arachnoid adhesions following in utero hemorrhage, infection, or trauma.9,10,11,12,13
On histologic examination, the cyst wall is lined with collagen and cells of the arachnoid matter (meningothelial cells). Electron microscopic findings confirm that the origin of these cysts is arachnoid cells and not epithelial cells.14 Immunohistochemical markers have also been used to differentiate arachnoid cysts from epithelial cysts. The cells lining the arachnoid cyst are not ciliated and do not stain with antibodies against glial fibrillary acidic protein (GFAP), S-100, transthyretin, and carcinoembryonic antigen (CEA).15
Arachnoid cysts usually present as isolated lesions. Several central nervous system (CNS) anomalies have been described in association with them. These include agenesis of the corpus callosum, absent septum pellucidum, deficient cerebellar lobulation, Arnold-Chiari type I malformation, malformations of cortical development, and arteriovenous malformation.16 We believe interhemispheric cysts that present in fetuses with commissural anomalies are probably not true arachnoid cysts but rather cystic processes that develop from an abnormal meninx that cause by themselves the defect, as they block the pathway of the axonal fibers through the midline. Non-CNS malformations associated with arachnoid cysts are tetralogy of Fallot, sacrococcygeal tumor, and neurofibromatosis type I.11,12,17,18,19 In addition, a few case reports point to the possible association of arachnoid cysts with nonchromosomal syndromes, such as distichiasis-lymphedema and Mohr syndrome, as well as trisomy 12q24.31.20,21,22 However, the strength of this association is questionable and may suffer from publication bias due to the limited number of case reports.
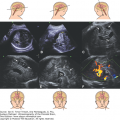
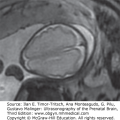
Generally, the finding of an arachnoid cyst is not thought to represent an increased risk for future pregnancies. However, arachnoid cyst is associated with a few hereditary conditions that confer increased risk of recurrence mostly in an autosomal recessive or an X-linked pattern. Aicardi syndrome, for example, is a rare X-linked dominant syndrome that includes agenesis of the corpus callosum, interhemispheric cyst, choroidal anomalies, and infantile seizures (Figure 11–1A).
Figure 11–1.
Arachnoid cyst at 16 postmenstrual weeks. (A) Axial plane showing the 0.7 × 0.54 cm cystic structure between the frontal lobes of the hemispheres. (B) A midcoronal section localizes the cyst between the hemispheres in the longitudinal sulcus. The exact localization of this arachnoid cyst was in the suprachiasmatic area. In spite of the favorable counseling, the patient elected for termination of the pregnancy. (Courtesy of Leibovitz Zvi Haifa, Israel.)

Most of the arachnoid cysts are sonographically detectable in the second or third trimester, which is consistent with the belief that these lesions only develop around this time. Prenatal diagnosis before 20 weeks’ gestation is uncommon. In one of the largest series evaluating fetuses with arachnoid cysts, there were no cases diagnosed prior to 20 postmenstrual weeks. Moreover, in this series 55% of the arachnoid cysts were diagnosed between 20 and 30 postmenstrual weeks and 45% only after the 30th week despite an earlier scan.2 Nevertheless, first trimester sonographic diagnosis coupled with histologic confirmation of an arachnoid cyst has been described (Figure 11–1).1,23
The characteristic sonographic appearance is that of a sonolucent cystic mass with a thin, smooth wall (Figures 11–2, 11–3, 11–4, 11–5, 11–6, 11–7, 11–8, 11–9). Arachnoid cysts do not communicate with the lateral ventricles (unlike some of the intraparenchymal cysts or lesions, such as porencephaly, dorsal cysts of holoprosencephaly, and schizencephaly).9,10 In severe cases, secondary hydrocephaly may be the result of an obstruction in the flow of CSF due to the mass effect. Most arachnoid cysts are supratentorial, with ~50% to 65% located in the middle cranial fossa, 5% to 10% in the suprasellar cistern, 5% to 10% in the quadrigeminal cistern, 5% spread along the convexities, and only 5% to 10% in the posterior fossa at the level of the cerebellopontine angle and the cisterna magna. Pierre-Kahn and Sonigo2 published the largest series including 54 patients with arachnoid cysts. Based on their experience, 63% of the cysts were supratentorial, mostly intrahemispheric (25%), with few in the base, suprasellar, or interventricular area. They found 22.2% of the cysts to be located at the infratentorial area.2
Figure 11–2.
Interhemispheric arachnoid cyst (*) associated with agenesis of the corpus callosum in a fetus at 22 weeks of gestation. (A) Axial view showing typical ventricular colpocephaly with a sharp shape of the anterior horn (arrow). (B) Coronal view. The interhemispheric fissure is continuous with the arachnoid cyst due to agenesis of the corpus callosum. (C) Median plane fails to show the corpus callosum (arrows); note also the presence of a severely dysgenetic vermis (arrowhead).

Figure 11–3.
Transvaginal brain study of a fetus at 25 postmenstrual weeks showing a quadrigeminal cistern arachnoid cyst. (A) Serial coronal sections from frontal–1 to occipital–2 (F-1 to O-2), with F-1 and frontal–2 (F-2) section showing the parenchyma of the frontal lobes. Midcoronal–1 (MC-1) to midcoronal–3 (MC-3) shows the midline anechoic cyst. In occipital–1 (O-1), the cyst can be seen slightly impinging on the posterior lobe of the brain. (B) Median (Med) and right and left oblique (Rt. and Lt. Obl) sections showing the extended and exact location of the cyst. Right oblique–1 and –2 (Rt. Obl-1 and -2) show the normal right cerebral hemispheres. Note that there is no dilation of lateral ventricles. On the median section, the arachnoid cyst is seen below the tail of the corpus callosum extending almost to the cranial bone. On left oblique–1 (Lt. Obl-1), more of the cyst is seen extending all the way to the outer surface of the brain. This fetus has a normal corpus callosum; therefore, the prognosis is good.

Figure 11–4.
Images of a large quadrigeminal plate arachnoid cyst at 26 postmenstrual weeks. (A) Median section showing the displacement of a quadrigeminal plate (QP) and the vermis of the cerebellum (V). (B) Midcoronal section showing the symmetrical position of the cyst displacing the hemispheres.

Figure 11–5.
Bilateral cysts at 32 postmenstrual weeks. The working diagnosis based on the sonographic evaluation was bilateral choroid plexus cysts. (A) “Horizontal”” section. The cysts are marked by arrows; the right is larger than the left. (B) Posterior angled “horizontal” section. The arrow points to the larger right–sided cyst. (C) Left oblique–1 section. Note the slightly dilated posterior horn and the smaller cyst (arrow) below the choroid plexus. (D) Midcoronal–1 section. Note that the anterior horns are slightly dilated. (E) Midcoronal–2 section. The cyst on the right side is shown. T, thalamus. (F) Occipital–1 section. The larger right-sided cyst is seen extending posteriorly even on this section. C, cerebellum. The neonate was born at term. The MRI images suggested the diagnosis of arachnoid cyst rather than cyst arising from the choroid plexus. (G) Slightly right paramedian section notes the thin wall of the cysts in the right lateral ventricle. This plane was obtained along the white line transecting the brain in Figure 11–5H. (H) “Horizontal” section. Note that the anterior horns (AH) are not dilated and that the right thalamus (T) is pushed slightly forward. (I) Coronal section through the two cystic structures and the cerebellum (C).

Figure 11–6.
Interhemispheric arachnoid cyst at 21 postmenstrual weeks affecting the corpus callosum. (A) Three-dimensional (3D) orthogonal planes: coronal (box A), sagittal (box B), axial (box C). Box D displays inversion rendering of the lateral ventricles (L) and the arachnoid cyst (arrow). (B) 3D power Doppler study. The planes are the same as in Figure 11–6A. Box D displays the short pericallosal artery (arrow).

Figure 11–7.
3D tomographic representation of the interhemispheric arachnoid cyst. Same case as in Figure 11–6. (A) Sagittal planes along the lines seen in the first box. (B) Coronal planes along the lines seen in the first box. (C) Axial planes along the lines seen in the first box.



Figure 11–8.
An arachnoid cyst originating from the quadrigeminal plate at 23 postmenstrual weeks. (A) The septated cyst displacing the falx to the right is shown by the coronal (box A), sagittal (box B), and axial (box C) planes. (B) Another set of orthogonal planes were selected to study the extent of the lesion. (C) Color Doppler study. The picture in the active box (box B) highlighted by the frame is generated in the median plane (dotted vertical white line through boxes A and C). Only the anterior cerebral artery, but not the pericallosal artery, is seen (the latter should be evident in this plane). (D) The plane is moved to the right. Now the active box (box B) is generated in the right parasagittal plane. In this plane, the pericallosal artery is clearly detected. This proves that the corpus callosum is not destroyed by the cyst. The dotted vertical white line was kept to mark the median plane. (E) A 3D color flow rendering of the anterior cerebral and pericallosal arteries. The approximate place of the arachnoid cyst is marked.

Figure 11–9.
Interhemispheric arachnoid cyst. (A) Two side-by-side coronal views of the cyst. Note the thin cyst wall (arrow) that depicts the diagnosis. (B) Although some views raised the suspicion of agenesis of the corpus callosum, this parasagittal image (especially some radially oriented gyri) shows the presence of a laterally displaced but normal-appearing corpus callosum (arrow). (C) 3D coronal tomographic sequence of the cyst. (D) 3D sagittal tomographic sequence of the cyst. (E) 3D axial tomographic sequence of the cyst. In addition to the above mentioned possible differential diagnosis of a partial agenesis of the corpus callosum we also considered schizencephaly as an alternative diagnosis. A postnatal MRI confirmed the diagnosis of arachnoid cyst parting the two hemispheres.

The differential diagnosis of an arachnoid cyst includes other extra-axial lesions, as well as intraparenchymal or intraventricular cystic lesions, as displayed in Table 11–1. With the exception of brain cystic tumors, which may be heterogeneous and contain solid components, all other lesions are sonolucent fluid cystlike masses that can be associated with secondary hydrocephaly due to a mass effect. In experienced hands, ultrasound (US) can distinguish between the different groups of intracranial cysts. For example, porencephalic cysts can be seen in the brain parenchyma and communicate with the ventricles and subarachnoid space.
Malformations of the vein of Galen have turbulent flow, which becomes evident using two- (2D) or three-dimensional (3D) color or power angiography.24,25,26,27 The sonolucent structure of the dilated vein of Galen does not communicate with the ventricles or the subarachnoid space and is located in the area of the quadrigeminal plate cistern (Figures 11–10 and 11–11). The aneurysm probably represents a remnant of the embryonic median prosencephalic vein. The diagnosis is generally made postnatally, and the clinical signs include cyanosis, systolic murmur, cardiomegaly, and increased pressure with hydrocephaly due to obstruction of the -sylvian aqueduct by the dilated aneurysm. In severe cases, nonimmune hydrops fetalis may result from severe high-output congestive heart failure. Gerards and colleagues28 described their experience with two cases of aneurysm of the vein of Galen. Their experience suggests that Doppler studies, 3D sonography, and magnetic resonance imaging (MRI) may be used to evaluate for prognostic factors, such as drainage and secondary damage.
Figure 11–10.
Aneurysm of the vein of Galen at 33 postmenstrual weeks. A–C. C-coded Doppler images of the lesion (arrows). (A) The median plane. (B) The midcoronal–1 plane. (C) In the median plane, note the wide (~1.2 cm) structure above the cerebellum (C) with flow toward the posterior pole of the brain. The Doppler evaluation showed a pulsating venous flow. (D) Grayscale image in the median plane. (E) Four-chamber view of the heart showing relative cardiomegaly; heart-to-chest diameter ratio: 0.65 (normal 0.45–0.5) and a very small amount of pericardial effusion (arrow). There was no atrioventricular valve regurgitation, but the heart had hyperdynamic motion consistent with a cerebral arteriovenous malformation. There was no dilation of the vessels in the neck of the fetus. Three attempts at embolization were performed after the delivery, but the neonate died during the last such attempt.

Figure 11–11.
Aneurysm of the vein of Galen at 22 postmenstrual weeks. (A) Two-dimensional (2D) grayscale median plane. (B) 2D color Doppler median plane. (C) 2D power Doppler median plane. (D) 2D power Doppler median plane of a normal vascular pattern for comparison. (E) Serial coronal 2D grayscale images of the pathology. (F) Serial coronal 2D power Doppler images of the pathology. (G) 3D grayscale multiplanar views of the pathology. The arrows point to the dilated vein.




Fetal brain tumors are extremely rare, have a heterogeneous pattern, are usually within the brain parenchyma, and may communicate with the ventricles.11 Teratomas are probably the only tumor that may appear as a completely cystic intracranial extra-axial neoplasm. Cassart et al29 published their experience with US and MRI of fetal intracranial tumors. Of 13 teratomas in their series, 12 had a significant cystic component, and few were completely cystic. These cystic components correspond to necrotic lesions and were extremely uncommon in other types of tumors. In a different series of fetal brain tumors, the authors diagnosed seven cases of brain tumors, of which six were confirmed postnatally. One case of a supratentorial arachnoid cyst was mistaken for a teratoma with cystic components. Out of the six cases of suspected teratomas, one was revealed to be a glioblastoma, one an arachnoid cyst, and one a primitive neuroectodermal tumor. The authors concluded that prenatal ultrasonography is a useful tool to identify any intracranial space-occupying lesion >10 mm, with 86% specificity. As expected, the accuracy of US in diagnosing the tumor’s histologic type was limited (57%).30 For a more detailed discussion and images of fetal CNS tumors, see Chapter 13.
Other extra-axial cysts, such as glioependymal and endodermal cysts, are extremely rare and cannot be distinguished sonographically from arachnoid cysts. Glioependymal cysts, also called ependymal cysts, choroidal epithelial cysts, neuroepithelial cysts, and epithelial cysts, have a glioependymal lining that distinguishes them from arachnoid cysts histologically. They may be located both extra-axially and intraparenchymally and have been reported to be detected in association with agenesis of the corpus callosum.31 This lesion is thought to arise from displaced neuroectodermal tissue most closely resembling the area forming the tela choroidea and may also have a heterogeneous sonographic appearance. Like arachnoid cysts, these lesions can grow and reach significant proportions.31 Several reports describe the detection of glioependymal cysts in fetuses undergoing neurosonographic evaluation or brain MRI for the evaluation of ventriculomegaly.32,33 The authors stressed the importance of considering this entity in the differential diagnosis of fetal cystic brain lesions, especially when callosal abnormalities coexist. Endodermal cysts are extremely uncommon congenital lesions that are rarely diagnosed prenatally. Most cases are located in the spinal canal, and of the intracranial ones, most are located in the posterior fossa.34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree