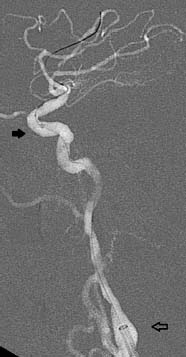
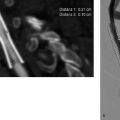
12 Intracranial Stenoses Worldwide, intracranial atherosclerotic stenoses are the most frequent cause of strokes in general. This epidemiologic significance is due primarily to the high prevalence of intracranial stenoses in Asia. In Europe and North America, by contrast, only 6–10% of all stroke patients are found to have a narrowing of intracranial vessels (Sacco et al. 1995; Weimar et al. 2006). Nevertheless, the high overall incidence of stroke, at 182 per 100 000 population, results in a significant number of affected individuals in Europe and North America too, and it is important to find an optimum treatment for these patients. Drug therapy with aspirin alone has an overall risk of stroke recurrence of —12% per year for all patients and up to 20% for individuals with high-grade stenoses. The recently published results of the SAMMPRIS trial proved that intensified conservative treatment effectively reduced recurrences to 12% in the latter ”high-risk” subgroup also (Chimowitz et al. 2011). For patients experiencing new ischemic events despite an optimized drug regimen, alternative treatment options are warranted. The EC/IC Bypass Study found that surgical revascularization with an extra- or intracranial bypass did not improve the prognosis (EC/IC Bypass Study Group 1985). Approximately 10 years after publication of the negative results of that study, initial case reports were published on intracranial angioplasty. Since then, the endovascular treatment of intracranial stenoses has been increasingly practiced and is now offered as a treatment option at almost all interventional neuroradiologic centers. High technical success rates are being achieved through the further development of the available materials. This chapter reviews the circumstances in which the modern endovascular treatment of intracranial stenosis can be of clinical benefit in selected patients using appropriate techniques. The interventional endovascular treatment of a symptomatic intracranial stenosis is a secondary prophylactic procedure done to minimize the risk of repeated stroke events. It must be demonstrated that the overall cumulative risk, consisting of strokes occurring as procedural complications plus recurrent strokes after technically successful stent therapy, is significantly less than the spontaneous risk with medical treatment alone. The WASID (Warfarin-Aspirin Symptomatic Intracranial Disease) trial tested and compared the efficacy of aspirin and warfarin in the secondary prophylaxis of symptomatic stenoses. Besides the fact that anticoagulants provided no benefit over antiplatelet drugs, it was found that the 1-year cumulative risk of recurrent stroke was 12% despite medical therapy (Chimowitz et al. 2005). A subgroup analysis of the same trial showed that patients with a high-grade intracranial stenosis had an almost 20% risk of a subsequent cerebral ischemia (Kasner et al. 2006). Analyses of published single- and multicenter case series on intracranial stenting indicated a procedural morbidity and mortality ranging from 7.7 to 9.5% (Cruz-Flores and Diamond 2006; Gröschel et al. 2009). The estimated risk of recurrent stroke after successful treatment is as high as 5.1% (Levy et al. 2007). The first device developed and approved for interventional treatment of intracranial atherosclerotic disease was the Wingspan stent system (Boston Scientific, San Leandro, CA, USA). From approval studies and subsequent registries a 1-year stroke risk of 15% was assumed for patients treated with Wingspan. Based on the results of the WASID trial and registry data, a randomized trial (SAMM-PRIS) was initiated to test ”best medical treatment” vs. Wingspan stent angioplasty in patients with high-grade (> 70%) symptomatic stenoses assuming an absolute risk reduction of 5% in the endovascular treatment arm. For this trial noninvasive management was intensified with a dual antiplatelet regimen during the first 3 months, rigorous control of all vascular risk factors, and lifestyle modification. Unexpected high procedure-related complication rates in the endovascular arm and astonishingly few recurrent strokes under medical treatment alone led to early termination of the trial. The 30-day morbidity and mortality was as high as 14.7% with endovascular treatment and 5.8% with medical treatment. Details of the procedure-related adverse events have not yet been published and the follow-up of enrolled patients is not complete, so a final judgment is not possible at the moment. However, until more details are released, a risk-benefit analysis would prohibit the uncritical use of intracranial angioplasty. Interventions should be reserved for selected patients who do not respond adequately to medical treatment. An analysis of the data from the GESICA (Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiac en Argentina) trial identified clinically significant hemodynamic stenosis as a risk factor for stroke recurrence (Mazighi et al. 2006). Hemodynamic compromise can be an argument for early treatment because this factor is not influenced sufficiently, or not rapidly enough, by antithrombotic treatment. In addition, these patients were probably not randomized to a large extent in the SAMMPRIS trial due to a high probability of new ischemic events during the randomization process. Note • The interventional treatment of symptomatic intracranial stenoses may be considered in cases of failed secondary medical prophylaxis. • First-line interventional treatment of high-grade symptomatic intracranial stenoses may be offered to patients with hemodynamically relevant luminal narrowing under the humanitarian device exemption. • The interventional treatment of asymptomatic intracranial stenoses cannot be recommended on the basis of current risk-benefit considerations. • The interventional treatment of intracranial stenoses should only be done in specialized centers with high expertise in endovascular management of atherosclerotic disease, to keep complication rates as low as possible. As in any surgical or interventional procedure, the patient must provide informed consent. This process should allow adequate time for reflection and should include the disclosure of general and specific procedural risks, alternative therapies, and special patient instructions regarding drug administration and follow-up. The most important aspect of informed consent, and the most relevant for the patient, is the disclosure of acute complication rates. Based on a systematic analysis of published case series and the results of the SAMMPRIS trial, the procedure-related stroke or death rate lies between 7.7 and 14.7%. Apart from these average numbers, all operators are obliged to assess their institutional morbidity and mortality rates, especially in the light of the SAMMPRIS results. These highly significant events overshadow any potential angiographic complications relating to vascular access or contrast administration, but the latter risks are still important and should also be disclosed. Only a few stents and balloon catheters have been approved for the treatment of intracranial stenoses. In theory, there are many more materials with properties that would make them suitable for use in the intracranial circulation. The individual vascular anatomy of the patient as well as complications may necessitate the use of unapproved materials, and patients as well as local boards and patients as well as local boards should be informed about the use of off-label products according to national regulations. As for patient instructions after the procedure, the need for compliance with medication should be emphasized. This is necessary to prevent acute thrombotic occlusion after stent placement with possible ischemic complications. In addition to medication compliance, regular follow-up examinations are recommended because up to 30% of patients develop restenosis. Practical Tip Points to remember during the informed consent procedure: • Humanitarian device exemption in patients with failure of medical treatment or hemodynamically relevant stenoses • Procedure-related stroke and death rate is 7.7–14.7%; institutional complication rates should also be assessed and communicated • Off-label use of angioplasty materials may be necessary • Restenosis rates are up to 30% • Compliance with antiplatelet therapy must be emphasized Once a patient has been selected for the interventional treatment of an intracranial stenosis, the optimum timing of the procedure needs to be considered. In the WASID trial, the subsequent stroke risk was significantly greater for patients enrolled less than 17 days after the acute transient ischemic attack (TIA) or stroke than for patients enrolled later (Kasner et al. 2006). Thus, the risk of recurrent stroke appears to be particularly high a short time after clinical presentation. This is an argument for early intervention in symptomatic patients, analogous to the management of extracranial stenoses. On the other hand, case series have shown a higher complication rate in patients treated during the acute phase up to 10 days after the initial event; this suggests that timing the intervention too early may affect the outcome just as adversely as a delay in treatment (Nahab et al. 2009). Possible explanations for higher complication rates in the acute phase are persistent thrombi, the instability of atherosclerotic plaques, and the risk of hemorrhage in large subacute infarctions. A pragmatic solution would be to schedule treatment at the start of the second week after the acute event. For high-grade hemodynamically significant stenosis presenting with progressive stroke, an expectant approach would of course be inadvisable and immediate action would be indicated. Dual antiplatelet therapy with aspirin (100 mg/day) and clopidogrel (75 mg/day) has proven essential in preparing patients for the stenting of supraaortic arterial vessels. Inadequate platelet inhibition increases the risk of stent thrombosis and thromboembolic events. For planned procedures, clopidogrel should be started 1 week before the intervention at 75 mg/day, as pharmacokinetically it requires several days for optimum effect. Alternatively, a higher loading dose may be administered the day before the procedure. A 600-mg oral dose of clopidogrel will produce adequate platelet inhibition after 4 h in most patients (Hochholzer et al. 2005). Aspirin, on the other hand, acts immediately after gastrointestinal absorption, so dosing may be started on the day before the intervention. Even if simple balloon angioplasty without a stent is planned, premedication with aspirin and clopidogrel is still recommended so that stent placement can be done safely in the event of an acute change of treatment plan or in an emergency (rescue stenting). Caution With the increasing use of intracranial stents, there has been growing interest in the problem of clopidogrel resistance. The efficacy of clopidogrel is subject to large interindividual variations, and up to half of patients have been classified as ”low responders” in different studies (Prabhakaran et al. 2008). The main reason for this is that clopidogrel is converted to its active form in the liver, and several enzymes of the cytochrome P450 group are involved in this process. The low activity of clopidogrel in certain patients may be genetically determined or may result from interactions with other substances that compete for cytochrome P450 enzymes. Laboratory tests are available for testing the efficacy of platelet inhibition and are increasingly used in interventional neuroradiology when stenting is scheduled. Unfortunately there is still a lack of definitive studies that can clinically validate the various tests and define accurate cutoff values. Laboratory tests do not offer absolute protection, however, and there are known cases of clinical non-responders who have developed stent thrombosis despite laboratory evidence of effective platelet inhibition. If clopidogrel resistance is found, doubling the dose will improve platelet inhibition in some cases. The platelet inhibitor prasugrel may be considered as an alternative. This drug has been studied in large numbers of cardiology patients and appears to be highly effective in preventing stent thrombosis. It should be added, however, that prasugrel therapy in patients with cerebral ischemic events was associated with an increased rate of incidents of major bleeding (Wiviott et al. 2007). It is still unclear whether the use of prasugrel will provide a net benefit in patients with symptomatic intracranial stenoses causing cerebral ischemia. Platelet inhibition is supplemented by intraprocedural intravenous heparin to prevent catheter thrombosis. The intravenous administration of 80–100 IU of heparin/kg body weight is generally sufficient. Anticoagulation should be checked by measuring the activated clotting time during angiography. The goal is to double the baseline value. It is unnecessary to continue heparinization after the procedure. Intracranial angioplasty requires careful catheterization of the peripheral cerebral vessels and accurate placement of the angioplasty material. General endotracheal anesthesia has proven effective for preventing patient movements during the procedure. There have also been case descriptions of intracranial stenting under regional anesthesia, but the great majority of interventions are performed under general anesthesia for the reasons noted above. Another advantage of treatment under general anesthesia is that it allows for swifter and more targeted action in the event of a complication. Practical Tip Special attention should be given to controlling arterial pressure during the procedure. A hemodynamically significant stenosis that has not yet been treated requires mild hypertension to ensure adequate cerebral blood flow. Immediately after vasodilatation, however, the arterial blood pressure should be returned to normotensive values to prevent a hyperperfusion syndrome. Arterial access to the target lesion is generally challenging as a result of vascular elongation, atherosclerotic wall lesions, and the occasional presence of more proximal stenoses in patients selected for intracranial angioplasty. The success of the procedure, in which the angioplasty material must be delivered far into the peripheral portion of the cerebral vasculature, depends critically on stable arterial access. Most materials used for stent angioplasty require at least a 6F guide catheter. This ”minimal” delivery system is recommended only for stenoses in relatively straight vascular segments in the intra- and extracranial circulation, as it provides only moderate proximal support. For treating intracranial stenoses in the anterior circulation, it is better to use a more stable 7F delivery catheter. In more difficult cases, an extra-long sheath or a combination of an extra-long sheath and guide catheter can be used as a coaxial system. Guide catheters with a flexible tip, known as distal access catheters, have also become available in recent years. They have an inner lumen that is large enough to accommodate angioplasty materials. The soft endpiece can be advanced far distally and even maneuvered through tortuous vessels without obstructing flow. When combined with a more proximally placed rigid sheath, this type of catheter system is a useful aid to successful completion of the procedure (Fig. 12.1). The vertebral arteries have small lumina that will tolerate only a small-caliber guide catheter. Proximal support can be provided by a long sheath that is left in the subclavian artery. With very difficult access conditions in the posterior circulation, an ipsilateral transbrachial approach may also yield the desired result. Fig. 12.1 Optimum stable distal access. The tip of a long sheath is within the carotid bulb (open arrow). A flexible-tip guide catheter has been passed through the sheath and advanced into the cavernous segment of the ICA (black arrow). This significantly shortens the intracranial vascular segments that need to be crossed to treat a stenosis of the middle cerebral artery (with kind permission of Dr. B. Turowski, Düsseldorf, Germany).
Introduction
Patient Selection
Informed Consent
Planning, Preparations, and Techniques
Scheduling the Procedure
Antiplatelet and Anticoagulant Therapy
Anesthesia
Arterial Access and Guide Catheter
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree