Class
Characteristics
Survival (months)
I
KPS 70–100
7.1
Age <65
Primary tumor controlled
Metastases to brain only
II
All others
4.2
III
KPS <70
2.3
Meningioma
Thirty-percent of primary intracranial neoplasms; twofold more likely in women (although incidence is equal for anaplastic meningioma s) and linked to ionizing radiation, viral infection, sex hormone s , NF2, and loss of chromosome 22q (Table 4.2).
Table 4.2
Simpson grading system for meningioma resection
Grade I
Macroscopic complete removal with excision of dural attachment, any abnormal bone, and involved venous sinus( es)
Grade II
Macroscopic complete removal with coagulation of dural attachment
Grade III
Macroscopic complete removal of intradural component(s), without resection or coagulation of dural attachment or extradural extensions
Grade IV
Partial removal with residual intradural tumor in situ
Grade V
Simple decompression with or without biopsy
Acoustic Neuroma
Acoustic neuromas (i.e., vestibular schwannoma s) arise from myelin sheath Schwann cells surrounding the vestibular nerve ; 6–8 % of intracranial tumor s, overall incidence ~1 % on autopsy studies.
Risk factors include acoustic trauma and coincidence with parathyroid adenoma; bilateral acoustic neuroma s pathopneumonic for NF2.
Both CN VII and VIII may be affected (hearing loss , tinnitus , vertigo , and unsteady gait), and extension into the cerebellopontine angle may lead to dysfunction of CN V (trigeminal pain ) and the facial nerve (facial paresis and taste disturbances), as well as compression of the posterior fossa (ataxia , hydrocephalus , and death ).
Mean growth rate ~2 mm per year, although may remain stable for years.
Paraganglioma
Rare neuroendocrine tumor s with incidence of ~1:1,000,000; sometimes called glomus tumor s or chemodectomas as they arise from glomus cells which function as chemoreceptors along blood vessels.
Can occur in the abdomen (85 %), thorax (12 %), and the head and neck (3 %); usually benign (<5 % malignant potential).
Pituitary Adenoma
Approximately 10 % of intracranial tumor s (5–25 % incidence on autopsy), almost all of which arise in the anterior lobe; 75 % functional (30–50 % prolactinoma , 25 % GH, 20 % ACTH , and <1 % TSH ).
Microadenoma <1 cm; macroadenoma ≥1 cm.
Presenting symptoms include headaches, hydrocephalus from 3rd ventricle obstruction , cranial nerve palsies with extension to the cavernous sinus , and bitemporal hemianopsia and/or loss of color discrimination from optic chiasm compression.
Forbes-Albright syndrome from prolactinoma : amenorrhea-galactorrhea in women, impotence and infertility in men.
Both mass effect and radiation damage to the pituitary infundibulum can cause an elevation in prolactin due to loss of hypothalamic inhibition (“stalk effect”).
Hormone levels typically normalize within 1–2 years after radiotherapy.
Arteriovenous Malformation (AVM )
Abnormal congenital communication between arterial and venous vasculature at a “nidus”; supraphysiologic hydrodynamic gradient.
Low incidence in the US population (0.14 %), but 8 % coincidence with cerebral aneurysm .
Annual rate of spontaneous hemorrhage ~2–6 %, with morbidity 20–30 % and mortality 10–15 % per event; after angiographic obliteration , lifetime risk of hemorrhage ≤1 %.
SRS induces vascular wall hyperplasia and luminal thrombosis, but requires several years to achieve full effect.
AVMs differ from cavernous malformations insofar as the latter are composed of sinusoidal vessels without a large feeding artery, and therefore have a low-pressure gradient (Table 4.3).
Table 4.3
Spetzler–Martin AVM grading system (1–5)
Size of nidus
<3 cm = 1
3–6 cm = 2
>6 cm = 3
Location
Adjacent to non-eloquent brain = 0
Adjacent to eloquent cortex = 1
Venous drainage
Superficial = 0
Deep = 1
Neuropathic Facial Pain
Trigeminal Neuralgia
CN V sensory nucleus disorder resulting in episodic, provokable (i.e., shaving, brushing teeth, wind, etc.), paroxysmal, unilateral, severe, lancinating pain lasting seconds to minutes in the distribution of the trigeminal nerve .
Predominantly idiopathic, although may be the result of trigeminal nerve compression by an aberrant artery or vein, or demyelination in multiple sclerosis. Secondary trigeminal neuralgia due to mass effect from meningioma , vestibular schwannoma , AVM , aneurysm , or other lesions.
Diagnosis of exclusion; obtain MRI to rule out cerebellopontine angle neoplasm.
Median time to pain relief after SRS is ~1 month; 50–60 % CR, 15–20 % PR; <10 % incidence of facial numbness after treatment.
Cluster Headache
Sudden onset of unilateral pain typically along the distribution of CN V1; associated with ipsilateral autonomic activity including ptosis , meiosis , lacrimation, conjunctival injection , rhinorrhea , and nasal congestion .
Etiology unclear; 6:1 male to female predominance.
GKRS to the trigeminal nerve alone not successful, and is associated with much higher rate of toxicity than during SRS for trigeminal neuralgia (Donnet et al. 2006; McClelland et al. 2006). Investigation of SRS to the pterygopalatine ganglion +/− trigeminal nerve root is ongoing (Kano et al. 2011; Lad et al. 2007).
Sphenopalatine Neuralgia (Sluder’s Neuralgia)
Rare craniofacial pain syndrome with 2:1 female predominance associated with unilateral pain in the orbit, mouth, nose and posterior mastoid process as well as ipsilateral autonomic stimulation from vasomotor activity .
Etiology unclear; perhaps related to pterygopalatine ganglion irritation from inflammation/infection of the sphenoid or posterior ethmoid sinuses.
Radiosurgical data limited to case reports of sphenopalatine ganglion treatment (Pollock and Kondziolka 1997).
Other
Small retrospective series of SRS for residual/recurrent pineal parenchymal tumors , craniopharyngiomas , and neurocytomas with high long-term local control and survival.
SRS used as salvage treatment for certain functional disorders, including epilepsy , Parkinson disease , and essential tremor with varying efficacy.
Stereotactic treatment of residual/recurrent glial tumor s , medulloblastoma , and other aggressive CNS malignancies has been reported, but outcomes are discouraging. Hypofractionation of recurrent glial tumors effective as salvage.
Treatment Indications
In general, SRS +WBRT is associated with longer survival than WBRT alone in patients with single metastases and KPS ≥70, improved LC and KPS preservation in patients with 1–4 metastases and KPS ≥70, and potentially, improved survival in patients with KPS <70.
SRS alone may provide equivalent survival and LC, plus improved neurocognitive outcomes when compared to SRS+WBRT or WBRT alone in patients with ≤3 metastases; close surveillance and salvage treatment is essential.
After resection, both SRS +WBRT and WBRT alone are acceptable adjuvant strategies, although SRS alone may be used in select cases with minimal intracranial disease and close surveillance (Linskey et al. 2010) (Tables 4.4 and 4.5).
Table 4.4
Radiosurgical treatment indications for brain metastases
Single lesion
Surgical resection + SRS to cavity
RPA class I–II
SRS alone for medically/surgically inoperable cases
2–4 Lesions
SRS +/− surgical resection with excellent prognosis/KPS
RPA class I–II
KPS ≤60, extensive intracranial/extracranial disease, and in combination with SRS as described above
WBRT
Table 4.5
Radiosurgical treatment indications for benign intracranial neoplasms
Meningioma
■ Recurrent/residual disease after surgery
■ Recurrent disease after prior SRS/ RT
■ Medically or surgically inoperable
Acoustic neuroma
■ STR (LF 45 % without adjuvant RT vs. 6 % with postoperative SRS)
■ Patient desire for greater preservation of useful hearing (30–50 % with surgery)
Pituitary adenoma
■ Adjuvant therapy after STR of macroadenoma with persistent post-operative hypersecretion or residual suprasellar extension
■ Consider medical management with bromocriptine or cabergoline for prolactin-secreting microadenoma
AVM
■ Medically inoperable, surgically inaccessible, or anticipated high morbidity due to Spetzler–Martin grade
Neurofacial pain
■ Failure of medical management (carbamazepine, phenytoin, gabapentin, baclofen, etc.)
■ Failure of surgical management (radiofrequency rhizotomy, balloon compression, microvascular decompression, etc.)
Workup
H&P with emphasis on neurologic components
Review of systems including any sensory changes , neurologic symtpoms, and endocrine abnormalities.
Laboratories:
No routine serum tests necessary for the evaluation of brain metastases, meningioma , AVM , neurofacial pain syndromes , etc.
Acoustic neuroma: Audiometry is the best initial screening, and typically shows sensorineural hearing loss (as will the Rinne and Weber test s ).
Pituitary adenoma s: Endocrine evaluation with prolactin , basal GH, serum ACTH , free cortisol , dexamethasone suppression, TSH , T3, T4, FSH, LH, plasma estradiol, and testosterone levels.
Imaging:
Thin-cut MRI with T1 pre- and post-gadolinium , T2, and FLAIR (fluid attenuation inversion recovery ) sequences; tumor enhancement after gadolinium correlates with breakdown of the blood-brain barrier , abnormal T2 signal indicative of gliosis and/or edema .
Can consider increased dose gadolinium at the time of radiosurgery to improve sensitivity of detection of brain metastases.
Hemorrhagic metastases most often seen with renal cell cancer, choriocarcinoma , and melanoma .
Magnetic resonance spectroscopy : tumors characterized by increased choline (cellularity marker), decreased N-acetylaspartic acid (NAA ; neuronal marker), and decreased creatinine (cellular energy marker); necrosis associated with increased lactate (anaerobic metabolism), and decreased choline/NAA/creatinine.
Dynamic magnetic resonance perfusion: relative cerebral blood flow (CBV) elevated in tumors (often in concert with grade), and decreased in areas of radiation necrosis and tumefactive demyelination .
Post-operative MRI should be performed within 48 h of surgery to document residual disease; acute blood appears as increased intrinsic T1 signal pre-contrast .
“Dural tail sign ” can be indicative of either tumor extension or vascular congestion associated with tumors adjacent or intrinsic to the meninges (seen with 60 % of meningiomas).
Meningiomas are isointense on T1 and T2, and intensely enhance with gadolinium ; evidence of bony destruction or hyperostosis in 15–20 % of cases. Acoustic neuroma: Seen as enhancing “ice cream cone” in the internal acoustic canal or as “dumbbell” projecting into the foramen magnum .
Pituitary adenoma s: X-ray skeletal survey should be performed in cases of acromegaly to evaluate growth plates
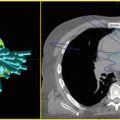
AVM : Co-registration of cerebral angiography and time of flight MRI sequences helpful for target delineation.
Neuropathic facial pain : Thin slice (1 mm) MRI /MRA has sensitivity and specificity of 89 and 50 %, respectively, for identifying vascular compression of the trigeminal nerve .
Radiosurgical Technique
Simulation and treatment planning.
Simulation with stereotactic frame in place.
Primary MRI planning with thin cuts (1–2 mm) preferred for intracranial radiosurgery , with fusion of preoperative scans if available.
If necessary, CT slices no thicker than 2 mm should be obtained and co-registered with MRI images.
Target volumes:
Brain metastases: GTV alone for intact lesions. For resection cavities, a 1–2 mm margin may increase local control (Soltys et al. 2008).
Meningioma, acoustic neuroma , pituitary adenoma, and other benign intracranial tumor s: GTV with 0–2 mm margin depending on degree of immobilization and stereotaxis.
Trigeminal neuralgia : Target ipsilateral trigeminal nerve adjacent to the pons in the retrogasserian cistern with a single, 4 mm shot. Retreatment isocenter should be located 2–3 mm away from initial target if possible.
Dose prescription:
See Table 4.6.
Table 4.6
Dose recommendations and outcomes for intracranial stereotactic radiosurgery
Presentation
Recommended dose
Outcomes
Brain metastases
■ 13–24 Gy/1 fraction depending on tumor volume/location
■ Dose reduction or hypofractionation (21–30 Gy/3–5 fractions) with larger lesions and/or resection cavities
■ Consider dose reduction (16 Gy) for brainstem lesions
Meningioma
■ Individualize dose based on tumor volume/location/surgical/radiosurgical history
■ 15 Gy/1 fraction for WHO grade I–III lesions; hypofractionation to 25–30 Gy/5 fractions possible, although long-term results unknown (UCSF experience).■ Grade III lesions may require higher dose
Long-term LC >90 % for WHO grade I lesions
Acoustic neuroma
■ 12–13 Gy/1 fraction
LC and preservation of CNs V and VII in excess of 95 %; hearing preservation ~75 %
■ 18–25 Gy/3–5 fractions
Appears safe and effective, but long-term results are unknown
Paraganglioma
■ 15 Gy/1 fraction or hypofractionation to 25 Gy/5 fractions
LC ~100 %
Pituitary adenoma
■ Nonfunctioning tumors: 12–20 Gy/1 fraction
■ Functioning tumors: 15–30 Gy/1 fraction (maximal safe dose); discontinue medical therapy 4 weeks prior to radiosurgery.
■ Single fraction optic apparatus tolerance: 8 Gy
■ 21–25 Gy/3–5 fractions
Appears safe and effective, but long-term results unknown
AVM
■ Individualize dose based on tumor volume; staged radiosurgery for larger lesions
2-Year obliteration rate for single-fraction treatment: <2 cm 90–100 %, >2 cm 50–70 %
■ 18 Gy/1 fraction for 8 cm3 target(s); dose escalation when feasible and safe (UCSF experience)
Trigeminal neuralgia
■ Primary: 70–90 Gy (100 % isodose line)
Pain relief in ~30–80 % of patients, although retreatment common; dose related to both relief from symptoms and development of new symptoms
■ Retreatment: 50–70 Gy (100 % isodose line)
Pineal tumors
■ Fractioned neuraxial RT for high-grade lesion; 15 Gy SRS reserved for residual tumor or local recurrence after RT
Consider hypofractionation in select cases if dose constraints to critical structures cannot be met with single-fraction treatment.
Dose delivery.
Multiple treatment modalities available, but most centers employ GK SRS , frameless robotic radiosurgery , and/or linac-based SRS.
Toxicities and Management
Stereotactic frame:
Mild headache immediately following frame removal, usually subsiding within 60 min.
Minimal bleeding from pin insertion sites requiring compression.
Peri-orbital edema resolving with head elevation and warm compress.
<1 % Risk of superficial skin infection.
Acute (1 week to 6 months):
Alopecia and skin changes following treatment of superficial lesions.
Mild fatigue .
Transient worsening of neurologic symptoms due to edema potentially requiring steroids .
Late (>6 months):
Radiation necrosis : Overall five-percent rate of symptomatic brain necrosis after SRS ; typically resolves with steroids , but may require surgical intervention.
Endocrine abnormalities.
Cranial nerve dysfunction following treatment of skull base tumor s.
Rare: memory impairment and cavernous malformations .
Isolated case reports of stroke , facial palsy /hyperesthesia , vision loss , and eye dryness after SRS for trigeminal neuralgia , all of which are very rare.
Recommended Follow-Up
Brain metastases and other high-grade lesions:
MRI 4–12 weeks after treatment, then every 2–3 months for the first 2-years, followed by imaging every 6 months for the next 3 years, and yearly thereafter; imaging intervals should be individualized according to clinical symptoms and lesion trajectory.
Low-grade lesions (meningioma , acoustic neuroma , paraganglioma, etc.):
MRI every 6–12 months for the first 2-years, then annually; imaging intervals should be individualized according to clinical symptoms and lesion trajectory.
Pituitary adenoma and other peri-sellar lesions:
Endocrine testing every 6–12 months with visual field testing annually.
Acoustic neuromas and cerebellopontine angle tumors:
Formal audiometry annually.
AVM :
MRI up to once per year for 3 years after treatment, with angiogram to confirm response after 3 years.
Neuropathologic facial pain and functional disorders:
Clinical follow-up only.
Evidence
Brain Metastases
SRS Boost with WBRT
RTOG 95-08 (Andrews et al. 2004): Randomized, multi-institution trial including 333 patients with 1–3 brain metastases and KPS ≥70 treated with WBRT (37.5 Gy/15 fractions) plus SRS (15–24 Gy/1 fraction) vs. WBRT alone. Significant survival advantage with SRS in patients with a single metastasis on univariate analysis (6.5 vs. 4.9 months), RPA class I on multivariate analysis (11.6 vs. 9.6 months), and trends for advantage with lung histology (5.9 vs. 3.9 months), and tumor size >2 cm (6.5 vs. 5.3 months). WBRT+SRS also associated with significantly higher 1-year LC (82 % vs. 71 %), and improved KPS (13 % vs. 4 %) with decreased steroid use at 6 months. Minimal acute- and long-term toxicity.
University of Pittsburgh (Kondziolka et al. 1999a, b): Randomized trial of 27 patients with 2–4 brain metastases and KPS ≥70 treated with WBRT (30 Gy/12 fractions) plus SRS (16 Gy/1 fraction) vs. WBRT alone. Study stopped early due to significant interim benefit in LC for WBRT+SRS (100 % vs. 8 %); median time to LF 6 months with WBRT vs. 36 months with WBRT+SRS. No difference in OS (8 vs. 11 months), and survival equal (~11 months) when accounting for SRS salvage in WBRT arm. No difference in OS or LC depending on histological type, number of brain metastases, or extent of extracranial disease.
SRS Alone or With WBRT
RTOG 90-05 (Shaw et al. 2000): Dose escalation study including 156 patients (36 % recurrent primary brain tumors, median prior dose of 60 Gy; 64 % recurrent brain metastases, median prior dose of 30 Gy). Maximum tolerated dose s of 24 Gy, 18 Gy, and 15 Gy for tumors ≤ 20 mm, 21–30 mm, and 31–40 mm in diameter, respectively; MTD for tumors <20 mm likely higher, but investigators reluctant to escalate further. Tumor diameter ≥2 cm significantly associated with increasing risk of grade ≥3 neurotoxicity on multivariate analysis ; higher dose and KPS also associated with greater neurotoxicity. Actuarial 24-month risk of radionecrosis 11 %. Patients with primary brain tumors and those treated on linear accelerator s (as opposed to GKRS ) had ~2.8-fold greater chance of local progression.
JROSG 99-1 (Aoyama et al. 2006): Randomized, multi-institution trial including 132 patients with 1–4 brain metastases (diameter <3 cm) and KPS ≥70, treated with SRS (18–25 Gy/1 fraction) vs. WBRT (30 Gy/10 fractions) followed by SRS. Trial stopped early due to low probability of detecting a difference between arms. Addition of WBRT reduced rate of new metastases (64 % vs. 42 %) and need for salvage brain treatment, and improved 1-year recurrence rate (47 % vs. 76 %). No difference in OS (~8 months), neurologic or KPS preservation, or MMSE score.
MDACC (Chang et al. 2009): Randomized trial including 58 patients with 1–3 brain metastases and KPS ≥70 treated with SRS (15–24 Gy/1 fraction) vs. SRS+WBRT (30 Gy/12 fractions) and followed with formal neurocognitive testing . Trial stopped early due to significant decline in memory and learning at 4 months with WBRT by Hopkins Verbal Learning Test (52 % vs. 24 %). However, WBRT also associated with improved LC (100 % vs. 67 %) and distant brain control (73 % vs. 45 %) at 1 year. Significantly longer OS with SRS alone (15 vs. 6 months), but patients in this arm received more salvage therapy including repeat SRS (27 vs. 3 retreatments).
UCSF (Sneed et al. 1999): Retrospective review of GKRS (n = 62) vs. GKRS+WBRT (n = 43); treatment characteristics individualized according to physician preference. OS (~11 months) and 1-year local FFP (71 % vs. 79 %) equivalent. Although brain FFP significantly worse for SRS alone (28 % vs. 69 %), no difference when allowing for first salvage (62 % vs. 73 %) after 1 year.
Sneed et al. (2002): Retrospective, multi-institution review of 569 patients with brain metastases treated with SRS alone (n = 268) vs. WBRT+SRS (n = 301); exclusion criteria included resection of brain metastasis and interval from end of WBRT to SRS >1 month. Median and overall survival no different among respective RPA statuses (I: 14 vs. 15 months; II: 8 vs. 7 months; class III: ~5 months). Twenty-four percent WBRT salvage rate in SRS patients.
EORTC 22951-26001 (Kocher et al. 2011): Randomized, multi-institution trial of WBRT (n = 81, 30 Gy/10 fractions) vs. observation (n = 79) following either surgery or SRS for 1–3 brain metastases in patients with stable systemic disease and ECOG performance status 0–2. Median time to ECOG performance status deterioration >2: 10 months with observation and 9.5 months with WBRT. OS similarly equivalent (~11 months), although WBRT reduced 2-year relapse at both new and initial sites. Salvage therapies used more frequently in the observation arm.
University of Cologne (Kocher et al. 2004): Retrospective review of patients with 1–3 previously untreated cerebral metastases treated with linac-based SRS (n = 117, median dose 20 Gy/1 fraction) or WBRT (n = 138, 30–36 Gy/10 fractions) stratified by RPA class. Rate of salvage WBRT: SRS group 22 %, WBRT group 7 %. Significantly longer survival after SRS in RPA class I (25 vs. 5 months) and class II (6 vs. 4 months) patients; no difference in RPA class III patients (4 vs. 2.5 months).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree