Intraoperative Image Update by Magnetic Resonance Imaging
Magnetic resonance imaging (MRI), in comparison to computed tomography (CT) and ultrasound, provides multiplanar imaging with a high soft-tissue resolution. It is generally accepted that MRI is the method of choice for the preoperative diagnostic evaluation of intracranial tumors. However, the closed-bore superconducting cylindrical design of MR scanners, with relatively long imaging times and difficult patient access, prevented their intraoperative application when MRI was introduced in clinical diagnostics. As a result, intraoperative imaging for evaluation of the resection completeness of a tumor was first investigated by ultrasound1–3 and CT,4–8 but the initial imaging quality and lesional resolution were not very satisfactory.
The development of MR systems with an open configuration has initiated the adaptation of these systems to the operating room (OR). Black and colleagues introduced a dedicated MRI system for intraoperative use in neurosurgery at Boston’s Brigham and Women’s Hospital.9 Their MR scanner was developed in collaboration with the General Electric Company. The GE scanner (0.5 Tesla Signa SP) has solved the problem of patient access in a cylindrical MR system with the so-called double doughnut design. The central segment of the cylindrical system is removed, allowing patient access through this vertical gap. An alternative to the design of a dedicated MR system for intraoperative use was our adaptation of an open MR scanner that was originally designed for diagnostic use only. The 0.2 Tesla Magnetom Open scanner (Siemens AG, Erlangen, Germany) was adapted for intraoperative implementation in cooperation with the Siemens company and the departments of neurosurgery at the Universities of Heidelberg and Erlangen.10–11 The unit has a biplanar magnet design that uses a resistive magnet. The C-shaped design with a horizontal gap allows wider access for patients.
The initial Erlangen concept,12 developed in 1994, was based on the installation of the MR scanner in a twin-operating theater in combination with two neuronavigation systems, which allowed intraoperative imaging in combination with frameless stereotaxy, so-called neuronavigation. Subsequently the Magnetom Open was installed in our OR in 1995. Soon thereafterafter, we incorporated magnetoencephalography (MEG) and functional MRI (fMRI), which allowed intraoperative identification of eloquent brain areas, a method also known as functional neuronavigation.13–16
Rubino et al17 were the first to report on the extension of this concept of surgery in the fringe field of the Magnetom Open scanner, which we had introduced for transsphenoidal procedures. They performed open cranium procedures directly on the MR table, with the head placed near the 5 Gauss line but without using additional neuronavigation guidance. In spring 1999, we began performing open cranium surgery in the fringe field of the MR scanner using the new NC4 navigation microscope (Carl Zeiss AG, Oberkochen, Germany), which could be operated in the low-magnetic field and rendered lengthy intraoperative patient transport unnecessary.18
To date, intraoperative MRI has been investigated in over 300 patients. Like other groups of investigators9,11,17,19–23 we have not observed any negative effects of intraoperative MRI. In our experience, intraoperative MRI is indicated in the surgical treatment of gliomas, especially low-grade gliomas, ventricular tumors, epilepsy,24 and complicated pituitary tumors. Furthermore, intraoperative MRI could be used to compensate for the effects of brain shift, if in complicated cases tumor remnants were to be localized in the surgical field and ongoing neuronavigational guidance was needed.25,26 Further indications, not yet investigated by our group, may be biopsy procedures with additional therapy control provided by the scanner (e.g., temperature monitoring in cryo- or laserablation).19,21 This chapter focuses on our experiences using intraoperative MRI for resection control in transsphenoidal pituitary surgery and in glioma surgery.
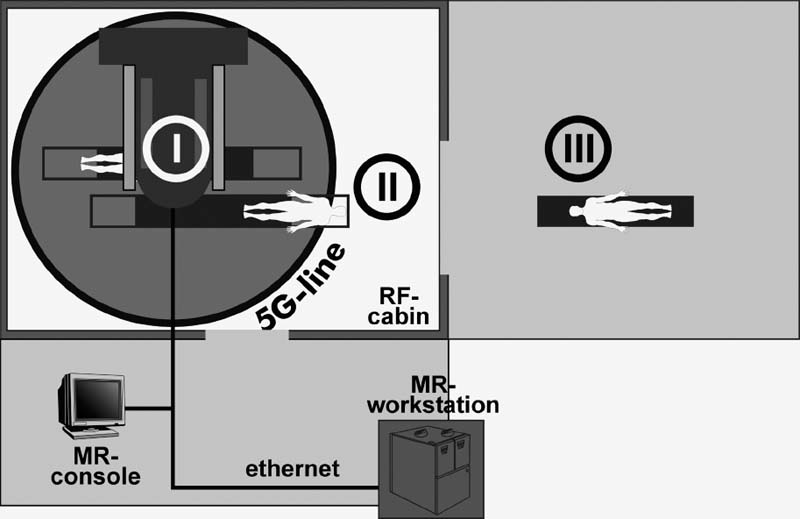
FIGURE 17–1. Operating theater setup with a 0.2 Tesla Magnetom Open MR scanner. There are three possible operating sites: (I) inside the scanner for interventional procedures, (II) at the 5 Gauss line for transsphenoidal surgery and craniotomy procedures using the NC4 navigation microscope, and (III) in the adjacent conventional operating room using the robotic MKM microscope (Zeiss, Oberkochen, Germany).
 Operating Room Setup for Intraoperative Magnetic Resonance Imaging
Operating Room Setup for Intraoperative Magnetic Resonance Imaging
The initial setup of the twin-operating theater (Fig. 17–1) included a conventional OR allowing surgery with magnetically incompatible instruments and microscopes. Two neuronavigation systems could be used in this conventional OR: a pointer-based system (Stealth-Station, Medtronic SofamorDanek, Boulder, CO, USA), and a microscope-based system (MKM, Carl Zeiss AG, Oberkochen, Germany). The MR scanner (0.2 Tesla Magnetom Open, Siemens AG, Erlangen, Germany) was placed in an adjacent radio frequency-shielded OR that was specially designed for the requirements of intraoperative MRI. With this setup the patient could be placed in three basic positions for surgery: (1) directly inside the scanner, used in interventional procedures such as biopsies only; (2) on the extended table of the MR scanner in the fringe field, which was initially used in transsphenoidal surgery or for catheter placements only; and (3) in the adjacent conventional operating theater, necessitating intraoperative patient transport. A specially designed air-cushioned OR table allowed intraoperative movement (over a distance of 4 to 5 meters) of a patient with an open cranium from the conventional OR to the MR scanner for imaging. This separation of operating site and imaging site was necessary because in 1995, microscope-based neuronavigation was available only in the form of the MR-incompatible MKM microscope. Since the introduction of the NC4 neuronavigation microscope, which can be used in the fringe field of the scanner, nearly all surgeries are performed in the radio frequency-shielded OR near the 5 Gauss line.18 Rather than lengthy intraoperative patient transport for imaging, the MR table itself is moved into the center of the MR scanner in less than half a minute.
 Intraoperative Magnetic Resonance Imaging in Transsphenoidal Surgery
Intraoperative Magnetic Resonance Imaging in Transsphenoidal Surgery
Until now, only anecdotal reports on the use of intraoperative MRI in transsphenoidal pituitary surgery were published.10,22,27–29 We investigated a series of 50 patients with large intra- and suprasellar, mainly hormonally inactive, pituitary tumors (44 adenomas, 6 craniopharyngiomas), which were operated using a transsphenoidal approach.30 The patient was lying directly on the MR table of the scanner; a standard flexible coil was placed around the patient’s head, which was embedded in a cushion. To obtain images in coronal and sagittal planes, T1-weighted spin echo sequences (slice thickness: 3 mm, TR: 340 ms, TE: 26 ms, FOV: 200 mm, matrix: 192 × 256) were measured. Optionally a T2-weighted turbo spin-echo sequence (slice thickness: 3 mm, TR: 5700 ms, TE: 117 ms, FOV: 230 mm, matrix: 224 × 256) was applied, allowing better delineation in cystic lesions and in cases where there was blood in the resection cavity.
In 72% of these 50 patients, intraoperative MRI allowed an ultra-early evaluation of tumor resection, which is normally only possible 2 to 3 months after surgery. A second look (i.e., a repeated inspection of the surgical field, n = 24) for suspected tumor remnants in the adenoma patients led to further resection in 15 patients (34%) (Fig. 17–2). However, image artifacts caused by metal debris from drilling or by blood accumulation in the resection cavity presented some challenges. Intraoperative MRI undoubtedly offered the option of a second look within the same surgical procedure if incomplete tumor resection was suspected. This improved the rate of procedures during which complete tumor removal was achieved. Furthermore, for those with incomplete tumor removal, additional treatments could be planned early—immediately following surgery. In contrast to intraoperative imaging, early postoperative MR studies performed a few days after surgery failed to show reliably the extent of the resection. In a significant proportion of cases, the extent of the mass lesion even exceeded its presurgery dimensions.31 Therefore both early CT and early MR investigations are unsuitable means to assess the extent of tumor resections. Intraoperative imaging is advantageous in that it is comparable to delayed postoperative investigations, which are generally accepted as the standard diagnostic postoperative procedures.
Intraoperative MR in transsphenoidal surgery is most indicated for large tumors with a distinctive suprasellar extension. In the case of drilling artifacts, which may impede proper image interpretation, it is possible to evaluate the extent of the suprasellar resection, but intra- and parasellar structures may not be easily identified. Immediately following the resection of a pituitary tumor a swelling of the cavernous sinus may occur due to the movement of the compressed sinus into the resection cavity. This can impede proper interpretation of the extent of intrasellar resection. Small intrasellar lesions or lesions that invade the cavernous sinus are not ideal candidates for intraoperative MRI because it is very difficult to differentiate between remaining tumor, normal pituitary gland, structures of the cavernous sinus, and blood remaining in the resection cavity. Perhaps in these difficult cases intraoperative sonography will be of further help. Furthermore, intraoperative Doppler sonography offers the possibility to visualize the relation to vascular structures in real-time mode.32–33
Restricted visibility of the supra- and parasellar tumor extension in transsphenoidal surgery initially led to the development of mirrors to enhance the visual field. Endoscopes used in transsphenoidal surgery34–35 further enhance the extent of the visual field and are a classic means of intraoperative imaging. But tumor remnants located in, for example, a suprasellar fold may not be visible, even with modern sophisticated endoscopic techniques. In these selected cases intraoperative MRI provides a reliable possibility to assess the extent of the tumor resection and may further improve the high efficacy of transsphenoidal microsurgery.
 Intraoperative Magnetic Resonance Imaging in Glioma Surgery
Intraoperative Magnetic Resonance Imaging in Glioma Surgery
All groups using intraoperative MR in recent years have shared the view that investigations in glioma patients are one of the main indications for intraoperative MR. Initial results published showed that the extent of tumor removal was increased by intraoperative MR23,36; even survival time seemed to be increased.23
For glioma surgery, our own group has combined intraoperative imaging with integrated functional neuronavigational support. On the one hand, MRI provides quality control to evaluate the extent of the resection; neuronavigation with integrated functional data, on the other hand, prevents too extensive resections, which would otherwise result in severe neurological deficits.37
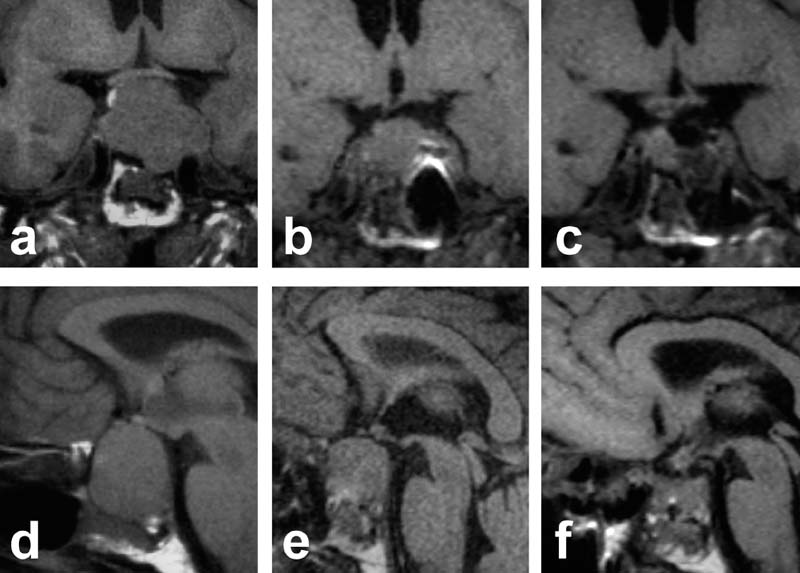
FIGURE 17–2. 51-year-old male with a large intra-, supra-, and parasellar, hormonally inactive pituitary adenoma. (A,D). Preoperative scans. (B,E). Intraoperative scans depict some remaining tumor. (C,F). After a second look and further tumor removal repeated imaging showed complete removal with preservation of the pituitary gland.
The locations of eloquent brain areas such as the motor cortex, the sensory cortex, speech-related areas, and the visual cortex were displayed intraoperatively in the neurosurgeon’s field of view using the heads-up display of the neuronavigation microscopes. To accomplish this “functional neuronavigation,” the functional data, which were obtained by MEG13–15 or fMRI,16 were integrated into the anatomical MR data set that was normally used for neuronavigation. All the functional data were registered with the anatomical three-dimensional (3-D) data set using a contour fit algorithm.38 The functional data were displayed in the anatomical dataset by markers inserted into the MR images. A pyramid or cube in the MR volume with white or black intensity accordingly represented a functional modality. The patient was registered with the navigation microscope (MKM or NC4-microscope with SMN-system, navigational software STP4.0; Zeiss, Oberkochen, Germany) at the beginning of surgery. The contours of the lesion, the predefined surgical approach, and the segmented markers for functional data were displayed using the heads-up display of the microscopes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree