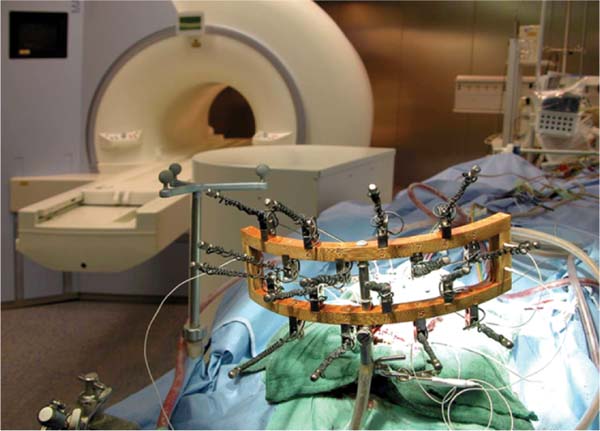
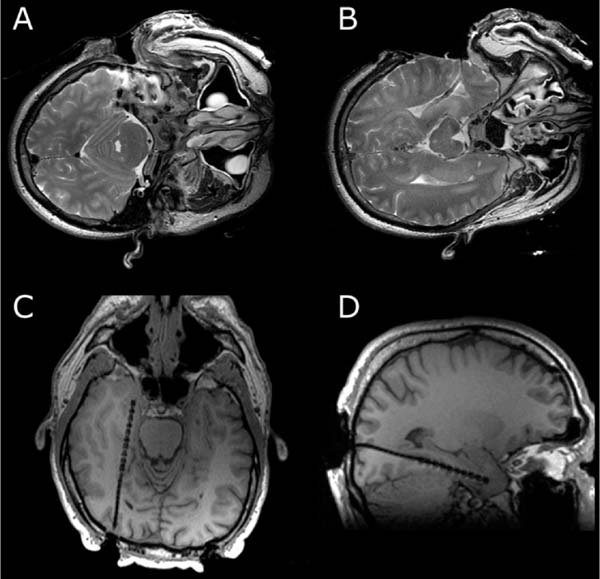
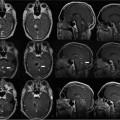
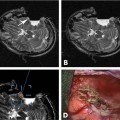
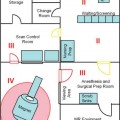
16 The basis of epilepsy surgery is a hypothetical focus, which is considered the origin of abnormal electrical activity within the brain that may or may not be associated with morphologic abnormalities. However, in many cases a lesion can be depicted by sophisticated imaging. Usually, pharmacoresistancy of seizures is required before an operation is considered and preoperative investigations are performed, which are hoped to reveal the origin and propagation of seizure activity.1 With the availability of morphologic (such as computerized tomography [CT] and magnetic resonance imaging [MRI]) and functional (such as interictal and ictal single photon emission tomography, positron emission tomography [PET], and magnetoencephalography [MEG]) imaging, epilepsy surgery underwent another enormous evolution. To date, a variety of operative procedures are designed to resect or disconnect an epileptogenic focus in the brain, so that no more seizures are generated or the abnormal electrical activity can no longer spread across the brain.2 Video electroencephalography (EEG) monitoring is usually the first step. It documents whether the patient actually suffers from epilepsy because it correlates the clinical manifestations of seizures to abnormal cortical discharges. Thereafter, a battery of structural and functional imaging methods are available that can reveal connatal or acquired lesions in the brain and areas of functional hyperactivity during convulsions or interictally. Based on these examinations a hypothesis is established as to where the abnormal discharges originate and how they spread throughout the brain. The aim of epilepsy surgery is either to resect this focus or to interrupt the propagation of abnormal electrical activity by disconnective operations. The cooperation of neurologists, neurosurgeons, psychologists, neuroradiologists, nuclear medicine specialists, and psychiatrists is very useful in this context. In any case, interdisciplinary cooperation should result in a well-structured treatment plan. With modern precise localization technologies being available, in many patients, foci such as unilateral mesial temporal sclerosis, slowly growing tumors typically associated with epilepsy, vascular anomalies, or cortical dysplasias are well defined in distinct areas of the brain. Recent developments in surgical techniques are targeted resections of what is believed to be epileptogenic tissues rather than the previously practiced standard resections of cerebral lobes. Whether a resection or disconnection actually accomplished the treatment plan was usually confirmed when postoperative MR images were obtained. Early attempts to utilize intraoperative MRI (iMRI) in epilepsy surgery to assess whether a tailored resection or disconnection procedure morphologically fulfills the individual needs of a patient were reported in 19983 and 19994 with the description of the systems and set-ups. The recently proposed concept of “as much as possible” limited tissue removal would support a more conservative resection than initially advocated by many centers to preserve tissue that is not necessarily epileptogenic or involved in propagation of the seizures. Its aim is mainly to reduce adverse neuropsychologic sequelae of brain tissue resection. Because a sophisticated MRI is one of the mainstays of a preoperative workup of an epilepsy patient who is prepared for surgery, it makes sense to depict the result of the operation also by MRI. Despite recent advances in epilepsy surgery5 that encompass the evolution of more-refined investigative techniques for the preoperative evaluation of patients with pharmacoresistant seizures and sophisticated operative procedures, further progress contributing to better delineation and definition of the epileptogenic process and its potential responsiveness to surgical resection is still eagerly awaited. In general, the extent of a resection is estimated by the surgeon at the time of the operation. However, a radiologic documentation of the amount and precise localization of resected brain was until recently only available several weeks after surgery. In this chapter, we review the experience gained with iMRI during surgery for epilepsy in patients with pharmacoresistant seizures. In 2000, we initially reported on the first 61 epilepsy surgery procedures with intraoperative low-field MRI utilizing a 0.2 tesla (T) MR (Siemens Magnetom; Siemens AG, Erlangen, Germany) system installed in our operating theater between 1996 and 2001.3 This had a shifting operating table on which the patient’s head could be brought just outside of the 5 gauss (G) line where it was unrestrictedly accessible and use of standard surgical instruments was possible. We initially even used a “twin operating theater” with patient transport on an air-cushioned system so that we could perform intraoperative electrocorticography (ECoG) in a standard shielded operating theater. Mean scanning time was 15 minutes.6 Nimsky et al7 implemented a navigation microscope within the fringe field of this MRI scanner, so that an update of navigation was possible utilizing intraoperative MRI data. A similar system with the same 0.2 T magnet, but with a tiltable surgical table was described by Lewin et al.8 They performed 21 operations for the treatment of seizures. Mean time required for their imaging sessions was 15.4 minutes for the intraoperative and 11 minutes for postoperative imaging sessions. The mean patient who had surgery for seizures underwent 1.67 imaging sessions. The Neurological Institute of New Jersey describe the use of their PoleStar N10 system (0.12 T; Medtronic Navigation, Inc., Louisville, CO) for assessing the extent of mesial temporal lobe resections in five patients.9 Likewise, Levivier et al27 provide a few pictorial examples of temporal lobe operations and excision of a left frontal cortical dysplasia with the PoleStar Medtronic system, in which the navigation tool of the equipment was also utilized. Walker et al10 reported on the Boston experience with the 0.5 T GE Signa system (General Electric Medical Systems, Waukesha, WI). There were 13 patients with benign intraaxial lesions presenting with seizures. The intraoperative set-up in the GE system, where the patient is lying in the MRI scanner during the whole operation, allowed the use of the MR imager as an online navigation device, but had the drawback that intraoperative electrophysiologic examinations (such as ECoG and hippocampal recordings) could not be performed in the vicinity of the system due to the high strength of the magnetic field. The ceiling mounted, mobile 1.5 T IMRIS system (IMRIS, Winnipeg, Manitoba, Canada) installed in Calgary in 1997 was also used for epilepsy surgery procedures. Kaibara et al11 reported on 14 patients with temporal lobe resections for pharmacoresistant seizures. They performed surgical planning and interdissection MRI in all 14 patients. Eight of the patients additionally had quality-assurance MR investigations. A set of coronal and axial images added 20 to 30 minutes to the operative procedure. Because this magnet can be moved away from the patient, ECoG can be performed in the same operating theater. A further analysis of the Calgary series experience with epilepsy surgery and iMRI comprising 70 patients by utilizing the 1.5 T high-field system was reported by Kelly et al.12 Neuronavigation was registered based on the preoperative planning images in 38 of the 70 patients (55%). Interdissection images were obtained in all but one patient (98.6%). They confirm that interdissection images led to further tissue resections in 26% of the patients. The currently used set-up in Erlangen was described by Nimsky et al.13 The “Brain Suite” consists of a 1.5 T MR imager equipped with a rotating operating table and located in a radiofrequency- (RF-) shielded operating theater. A navigation microscope placed inside the 0.5 millitesla (mT) zone and used with a ceiling-mounted navigation system enables integrated microscope-based neuronavigation. In this system, the patient moves. Draping the patient and rotating the operating table for scanning takes some 2 minutes. We have shown that intraoperative ECoG for epilepsy surgery is possible within the RF-shielded operating theater; in close vicinity to the MRI scanner (Fig. 16.1). During the 7.5 years between April 2002 and August 2009, 1424 operative cranial neurosurgical procedures were performed in this system and 170 of these were operations for epilepsy other than tumor resections. There were 12 implantations of electrodes, 156 resections, and two multiple subpial transections performed with 1.5 T MRI intraoperatively. One-hundred thirty temporal lobe resections and 25 extratemporal resections were performed. In 124 of the procedures, navigation was used based on intraoperatively acquired datasets. The improvements in workflow, image quality, and efficiency between the low- and high-field systems in the same department were analyzed by Nimsky et al.14 Despite the considerable advances in imaging technology and sophisticated functional investigations, there remain a substantial number of patients in whom the noninvasive evaluations fail to precisely localize the focus. Further information is frequently gathered by the implantation of subdural or intracerebral electrodes for a few days or by intraoperative ECoG. Although attempts have been made to reduce invasive diagnosis to a minimum, its availability in difficult cases characterizes a universal epilepsy surgery program. It is a general custom to implant subdural electrodes through burr holes under radiofluoroscopic control. Their position is thus only estimated in relation to bony landmarks of the skull because the x-ray does not depict the brain.5 Only grids are implanted via craniotomies and the position of electrodes is then directly visible on the brain surface. Direct cortical stimulation using these electrodes may reveal further information on functionally important regions. The intraoperative low-field MRI beautifully depicted the position of hippocampal and depth electrodes.6 We thus confirmed in the operating theater that the electrodes were placed where we wanted to record. If required, repositioning could easily be achieved and the positions rechecked. The high-field system is advantageous, particularly concerning the quality of the images. Figure 16.2 shows the basal subdural and hippocampal electrodes for intraoperative corticography, which allow for correlation between the neurophysiologic recordings and the topography of the brain. The figure also shows a depth electrode that records through the axis of the hippocampus. Mehta et al15 describe the advantages of frameless stereotactic placement of depth electrodes with a commercially available navigation system in epilepsy surgery. Implementation of a navigation tool that uses the MR data registration within the same operating theater and patient position in which the operation is performed seems to be advantageous because it avoids potential inconsistencies and thus improves the accuracy of electrode placement. Ideally, the resulting electrode localization can be immediately visualized, which is also possible with an iMRI. Depiction of subdural and intracerebral electrodes by MRI is of no safety concern.16 Fig. 16.1 Set-up for epilepsy surgery with intraoperative magnetic resonance imaging (MRI): electrocorticography with the Montreal system and reference star for neuronavigation (BrainLab VectorVision; BrainLab, Feldkirchen Germany) in the front; 1.5 T MRI scanner (Siemens Sonata, Maestro Class; Siemens AG, Erlangen, Germany) in the back. Fig. 16.2 Intraoperative localization of electrodes. (A) The subdural strips were placed for intraoperative electrocorticography. Their exact localization detected by intraoperative magnetic resonance imaging (iMRI) can be used for functional neuronavigation during surgery. (B) The hippocampal electrode was placed transcortically into the ventricle for intraoperative electrocorticography. The iMRI documents the correct placement and the anatomic relation to the hippocampus. (C,D) The deep brain electrode was placed with use of neuronavigation (“frameless stereotaxy”) into the hippocampus for chronic recordings. The iMRI documents the correct placement. Temporal lobe resections are the most frequently performed neurosurgical operations for the treatment of pharmacoresistant epilepsy. A major advantage of these procedures is that most of these can be undertaken without causing a neurologic deficit at least in most patients and particularly in the nondominant hemisphere. The concept of standard resections of the anterior temporal lobe survived for decades. One of the ongoing controversies is that of structure-function relationship, which was more recently approached by systematic sophisticated assessment of the extent of tissue resection in relation to the pre- and postoperative loss of cognition and to the efficacy of the procedure with respect to seizure control. The background of tailored anterior or posterior temporal lobe resections is an attempt to achieve more precise resection, thus maximizing preservation of normal brain. Various suggestions as to how to achieve this goal have been made in the past and a variety of different surgical procedures have been devised.5
Intraoperative Magnetic Resonance Imaging for Epilepsy Surgery
Systems and Set-ups
Intraoperative Recordings and Positioning of Electrodes
Temporal Lobe Resections
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree