This article describes the rationale, indications, and modality for intraoperative brain mapping for safe and effective surgical removal of tumors located within functional brain areas.
Intraoperative mapping refers to a group of techniques that allow for safe and effective removal of lesions located in so-called eloquent or functional areas. Although the entire brain can be inferred as eloquent, the eloquent areas are usually involved in motor, language, visual, or visuospatial control. The surgical removal of lesions in or close to those areas or pathways aims to maximize surgical removal and to minimize postoperative morbidity. These results can be achieved by the identification and preservation during surgery of the cortical and subcortical sites involved in specific functions. Detecting and preserving the essential functional cortical and subcortical sites have recently been defined as surgery according to functional boundaries, and is performed using the brain-mapping technique.
Brain-mapping techniques are generally applied to the surgical resection of intrinsic lesions, such as gliomas., They can occasionally be used during removal of cavernoma or meningioma, when these lesions are located in or close to functional areas of the brain. However, these techniques have been developed and used mainly for the surgical resection of low-grade gliomas.
Low-grade glioma refers to a series of primary brain tumors characterized by benign histology and aggressive behavior related to the slowly progressive invasion of the normal brain parenchyma. These neoplasms are classified as grade II (of IV) by the World Health Organization classification of brain tumors and include grade II astrocytoma (further divided into fibrillary and protoplasmic), grade II oligoastrocytoma and grade II oligodendroglioma. Pilocytic astrocytomas, or grade I astrocytomas, are occasionally referred to as low-grade gliomas but because of their peculiar behavior, they require separate considerations. Low-grade gliomas are slow-growing tumors, typically affecting younger individuals (median age 35 years), mainly men (male/female ratio 1.5), which clinically present with seizures (often partial seizures). Headache, personality changes, and focal neurologic deficits are the other most common symptoms. The neurologic symptoms include motor/sensory deficits, dysphasia/aphasia, disinhibition, apathy, and visuospatial disturbances, according to the tumor location and size. Low-grade gliomas mainly occur in eloquent areas or in their proximity, and grow diffusively, along short and long white-matter tracts. They grow continuously, and the speed of growth as measured on magnetic resonance (MR) images has been advocated as an important prognostic factor. In addition, these tumors systematically evolve toward a more malignant phenothype: so-called malignant transformation. Overall, the median survival of low-grade gliomas is about 10 years and well-defined negative prognostic factors include older age (>40 years old), larger size (>5 cm), eloquent location, and reduced Karnofsky performance status. The optimal treatment of those tumors that are no longer indolent has yet to be determined. Watchful observation, needle biopsy, open biopsy, and surgical resection have all been advocated by different investigators. Surgical resection of low-grade gliomas is still a matter of debate but recent studies have increasingly supported resection. Surgery can achieve multiple aims: a more reliable histologic diagnosis with eventual molecular profiling (eg, 1p/19q loss and methyl guanine methyl transferase [MGMT] status); symptom relief; a beneficial effect on seizure control; and a decrease in the rate of recurrence and of malignant transformation, as confirmed by recent studies. Nevertheless, surgery carries risks, which although low can permanently affect the patient’s quality of life. Considering the behavior of low-grade glioma and the possibility of treatment, the modern surgical approach to low-grade gliomas is to resect the tumor mass maximally, and at the same time to minimize postoperative morbidity to preserve the patient’s functional integrity. Because the natural history of the tumor can be long (with or without surgery), the conservation of simple and complex neurologic functions of the patients is mandatory.
High-grade gliomas are a larger group of intrinsic brain tumors, which include anaplastic tumors (such as oligodendrogliomas or astrocytomas) and glioblastomas. They can be classified as primary, when they are discovered and diagnosed de novo, or they can come from the transformation and evolution of low-grade gliomas. They are highly infiltrative neoplasms, invading along white-matter tracts and vessels. In addition, they are characterized by an elevated tumor cell proliferation, which results in the formation of a large, densely cellulated tumor mass, with high vascularization. Less frequently than low-grade gliomas, these tumors may develop within or close to eloquent areas or pathways. In such cases, as with low-grade gliomas, surgical removal should involve amaximal tumor removal, which is associated with the best oncologic result, while still maintaining the patient’s total functional integrity, which allows the patient rapid access to adjuvant treatments.
To achieve the goal of a satisfactory tumor resection associated with the full preservation of the patient’s abilities, a series of neuropsychological, neurophysiologic, neuroradiological, and intraoperative investigations have to be performed. This article describes the rationale, the indications, and the modality for performing safe and effective surgical removal of tumors located within functional brain areas.
Rationale and indications
The major aims of surgical treatment are: (1) to obtain adequate specimens and representative tissue to reach a correct histologic and molecular diagnosis; (2) to achieve a maximal cytoreduction to decrease the rate of recurrence and malignant transformation for low-grade gliomas, and increase the effect of adjuvant therapies for high-grade gliomas, in both cases possibly prolonging patient survival; (3) to improve the neurologic symptoms of the patients; and (4) to obtain better seizure control. These goals can be reached by tailoring the surgical approach to the particular features of location, modality of growth, and behavior of the tumor.
Surgery for gliomas aims to remove the tumor mass maximally and to preserve the patient’s functional integrity. This policy applies to the resection of any glioma but more specifically to those located close to or within eloquent areas. The concept of eloquence refers not only to those areas that are involved in motor, language, or visuospatial functions but also, more widely, to any area affecting the well-being of the individual (eg, memory, socioaffective behavior, specific task performance). In all these cases, extensive resection and maximal functional integrity can still be achieved through the intraoperative use of brain-mapping techniques.
The brain-mapping technique
Brain mapping requires a series of preoperative evaluations and intraoperative facilities that involve different specialists. A complete neuropsychological evaluation is generally the first step of the process, to select suitable patients and individualize intraoperative testing. In addition, sophisticated imaging techniques, including functional magnetic resonance imaging (fMRI) and diffuse tensor imaging, fiber tracking techniques (DTI-FT), help with surgical strategies. These images can be loaded into the neuronavigation system and are thus available peri- and intraoperatively for orientation. Intraoperative MR can also be used. A series of neurophysiologic techniques are employed at the time of surgery to guide the surgeon precisely in the tumor removal. These techniques include cortical and subcortical direct electrical stimulation (DES), motor evoked potentials (MEPs), multichannel electromyography (EMG), electroencephalography (EEG), and electrocorticography (ECoG) recordings. These techniques are detailed in the next section. For reasons of simplicity, the management protocol is divided into 3 parts: preoperative, perioperative, and postoperative.
The brain-mapping technique
Brain mapping requires a series of preoperative evaluations and intraoperative facilities that involve different specialists. A complete neuropsychological evaluation is generally the first step of the process, to select suitable patients and individualize intraoperative testing. In addition, sophisticated imaging techniques, including functional magnetic resonance imaging (fMRI) and diffuse tensor imaging, fiber tracking techniques (DTI-FT), help with surgical strategies. These images can be loaded into the neuronavigation system and are thus available peri- and intraoperatively for orientation. Intraoperative MR can also be used. A series of neurophysiologic techniques are employed at the time of surgery to guide the surgeon precisely in the tumor removal. These techniques include cortical and subcortical direct electrical stimulation (DES), motor evoked potentials (MEPs), multichannel electromyography (EMG), electroencephalography (EEG), and electrocorticography (ECoG) recordings. These techniques are detailed in the next section. For reasons of simplicity, the management protocol is divided into 3 parts: preoperative, perioperative, and postoperative.
Preoperative protocol
The preoperative part includes the neuropsychological and neuroradiological evaluation, which completes the standard neurologic examination. A neuroanesthesiologic evaluation should be performed also for the selection and preparation of the patients from this perspective.
Neuropsychology
Neuropsychological evaluation is composed of a large number of tests for the assessment of various neurologic functions, such as the cognitive, emotional, intelligence, and basic language functions. This broad-spectrum evaluation provides information on how the tumor has affected the social, emotional, and cognitive life of the patient, who is frequently intact or only mildly impaired at the neurologic examination. Test area should be as large as possible because a tumor that grows along fiber tracts may alter the connectivity between separate areas of the brain, resulting in the impairment of functions that might not be documented if the examination is limited to those functions strictly related to the area of the brain in which the tumor has grown. When this extensive testing is administered, some alteration in the neuropsychological examinations can be documented in more than 90% of patients with low-grade gliomas, and in more than 70% with high-grade gliomas. These data represent the baseline against which future treatment should be compared. In addition, when the tumor involves language or visuospatial areas or pathways, a more extensive specific evaluation should also be performed. In addition to better defining the preoperative status of patients, the neuropsychological assessment is a series of tests, composed of various items, which are used intraoperatively for the evaluation and brain mapping of various functions, of which memory, language, and visuospatial orientation are some of the most important. For language evaluation, patients are submitted preoperatively to extensive language testing from a battery of tests aimed at evaluating oral language production and comprehension, together with repetition. Hemispheric language dominance is evaluated through the Edinburgh Inventory Questionnaire and fMRI. The following tasks are usually performed: spontaneous speech; oral controlled association by phonemic cue; famous face naming; object picture naming; action picture naming; word comprehension; sentence comprehension; transcoding tasks. The token test, the digit span, and counting are also performed. Ideomotor apraxia and face apraxia are assessed. Most of the tests used have been standardized on the normal population. In addition, different tests aimed at studying the previous aspects of language can be found and adjusted according to the nationality of the patient. Qualitative and quantitative tests should be included in the battery, and normative data must be available for the quantitative procedure. A speech therapist and a neuropsychologist should manage the assessments. Preoperative language evaluation is used in a series of tests intraoperatively for the assessment of language; object naming is probably the most important. In patients with a tumor located in dominant or parietal areas, number recognition and reading, calculation, or writing should be added to the preoperative testing and considered for the intraoperative evaluation. When the patient is bilingual or speaks more than 2 languages, the preoperative testing should include extensive evaluation of the various languages. The patient can be defined as early or late bi- or multilingual, depending on the time at which he or she has learned the various languages. A multilingual assessment is generally recommended.
Visuospatial functions are usually evaluated for tumor located in the parietal lobe, generally on the right side. Unilateral spatial neglect is a complex and disabling syndrome that typically results from right-hemisphere damage, and it is characterized by an impairment of awareness of the contralesional left half of space, objects, and mental images. These patients are given various tests such as the line bisection test or the star cancellation test to evaluate their spatial awareness.
Imaging and Neuroradiology
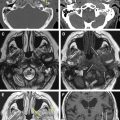
The neuroradiological examination is composed of basic examinations, such as morphologic T1, T2, and fluid attenuated inversion recovery (FLAIR) images, and postcontrast T1 images. These images together with volumetric sequences provide information on the site and location of the tumor, and allow determination of its relationship with various structures, such as major vessels, and measurement of tumor volume. With low-grade gliomas, additional MR studies such as MR spectroscopy or MR perfusion can be performed. MR spectroscopy, which provides information on the metabolic characteristics of the tumor, allows design of a map of areas within the tumor in which tumor metabolism is more or less pronounced (a multipixel MR spectroscopy map). This helps with tissue sampling during surgery for histologic and molecular purposes. In addition, perfusion MR studies are useful for designing perfusion maps, or maps in which the blood flow is depicted in the different tumor areas. Because regional blood flow is dependent on tumor angiogenesis, these maps provide additional and complementary information on the biologic behavior of the tumor and help with collection of tissue during surgery for histologic and molecular examination. Metabolic information may be also obtained by performing single photon emission computed tomography (SPECT) or positron emission tomography (PET), and these data may be incorporated into the navigation system for surgical guidance. PET with fluorodeoxyglucose (FDG) is generally used for high-grade gliomas, and metionine for low-grade tumors.
The neuroradiological investigations include functional studies, such as fMRI, and anatomic studies such as DTI-FT. The former provides functional information on the location of cortical sites that activates in response to motor tasks or various language tasks. Motor fMRI is generally used to design a map of the cortical motor sites and to establish their relationship with the tumor. fMRI for language provides a map of the cortical sites that activate during various language tasks, such as denomination (object naming), verb generation, and verbal fluency. All these data are pooled to form a complex map of how the various components of language are organized at the cortical level, and establish the spatial relationship between these cortical areas of language activation and the tumor mass. It is usually recommended that language fMRI is performed with the same tests that are used for language evaluation to increase its reliability.
The DTI-FT techniques allow the connectivity around and inside a tumor to be depicted, by reconstructing and visualizing the fiber tracts that run around or inside the tumor mass. A deterministic DTI-FT approach is generally used for clinical purposes. DTI-FT provides anatomic information on the location of motor tracts, mainly the corticospinal tract and various language tracts. The basic DTI-FT map includes the corticospinal tract (CST) for the motor part, and the superior longitudinalis (SLF), which includes the fasciculus arcuatus, and the inferior frontooccipital (IFO) tract for the language part. The SLF is the basic tract involved in the phonologic component of language; the IFO is the basic tract involved in the semantic component of language. Additional tracts that can be reconstructed are the uncinatus (UNC) and the inferior longitudinalis (ILF) tracts, which provide information on the semantic and phonologic component of language in the frontal and temporal lobe, or the subcallosum fasciculus, involved in the phonologic component of language, sited in the lateral border of the lateral ventricle. Generally, preoperative DTI-FT shows that in low-grade gliomas most of the tracts involved in language or motor function are located within the tumor mass, and infiltrated or interrupted by the tumor. In high-grade gliomas most of the tracts are located in the tumor periphery and dislocated by the tumor mass. Although DTI-FT maps are only anatomic and do not provide any functional information, they can be used to predict resectability of a tumor. Preoperative neuroimaging produces a large amount of information concerning the anatomic and functional boundaries of the lesion to be resected. Together with the volumetric morphologic images, the DTI-FT images are usually loaded into the neuronavigation system, and help in the perioperative period in performing the resection. However, the imaging gives information based on probabilistic measurements, and although they may have a high sensitivity or specificity, they still carry the potential for error, which is not efficient for performing a safe and effective resection. For this reason the neuroradiological information loaded into the neuronavigation system has to be supported during surgery by the results of the brain mapping. Only intraoperative brain mapping by electrical stimulation allows the surgeon to identify functional regions, which may be displaced and infiltrated by the tumor at a cortical and a subcortical level, and thus to define the strategy of resection to maximize the extent of tumor removal and reduce the risks of permanent neurologic deficits.
Anesthesiology
Besides the standard anesthesiological work-up, the patient should be examined for his or her capacity for intraoperative awake monitoring. Preparation and selection of the patients by anesthesiologists with expertise in surgery in an awake patient is recommended. In the authors’ institution, the only absolute contraindications to awake surgery are lack of cooperation from patients, patients older than 65 years, obese patients, patients with difficult airways, or patients affected by severe cardiovascular or respiratory diseases, common contraindications to any general anesthesia regimen, communication difficulties (moderate-to-severe aphasia), psychological imbalance (extreme anxiety), prone position, and inability to lie still for many hours.
Once the preoperatory work-up (according to the site and the characteristics of the tumor), the neuropsychological evaluation, and the functional and anatomic imaging are completed, each patient is offered an individualized surgical and monitoring strategy, which can be summarized as follows:
- 1.
Lesions in the nondominant hemisphere, away from eloquent areas and with no relationship with areas of activation according to fMRI: motor monitoring (optional).
- 2.
Lesions in the nondominant hemisphere, in central or precentral area or in relationship with the CST (eg, insular, temporomesial tumors) and small central lesions in the dominant hemisphere: motor mapping and monitoring.
- 3.
Lesions in the nondominant hemisphere, in the postcentral region: motor mapping and monitoring, visuospatial mapping.
- 4.
Lesions in the dominant hemisphere: motor mapping and monitoring, language mapping ± visuospatial mapping for parietal lesions.
Intraoperative protocol
The general policy in surgical treatment is to remove the maximal amount of tumor and to preserve the functional integrity of the patient, by removing the tumor according to anatomic and functional boundaries. The anatomic boundaries can be defined by using neuronavigation or intraoperative MR; sonography can also be used. The functional boundaries can be defined by using neurophysiologic and neuropsychological adjuncts. The intraoperative protocol includes anesthesia modalities, neurophysiology, neuropsychology, and intraoperative imaging (neuronavigation and intraoperative MR).
Anesthesia
The patient can be kept awake during surgery, or awakened for the phase during which the mapping is performed. Total intravenous anesthesia with propofol and remifentanil is used in the authors’ institution for performing these procedures. Newer drugs, such as dexmedetomidine, are emerging as effective and safe in achieving sedation without inducing respiratory depression or affecting electrophysiologic monitoring. In the authors’ institution, patients requiring only motor mapping are intubated through the nose and a light surgical anesthesia is maintained throughout the procedure. No muscle relaxants are employed during surgery to allow the neurophysiologic assessment. When the language or the visuospatial functions have to be tested intraoperatively, the patients receive a laryngeal mask, which is maintained until after dural opening. At this point, the patients are awakened, and adequate analgesia is maintained to allow functional monitoring. The anesthesiologist should keep the patient awake during the entire subcortical mapping, and particularly during long operations this may require alternating periods of resting with periods during which the patient is fully awake and responsive. Fatigue is observed in most patients, and its appearance correlates with the duration of the mapping and the difficulties of testing (extensive language and visuospatial mapping). Seizure is the most important complication during the awake time of the surgery, and can be controlled either by cold saline irrigation or by the infusion of a small bolus (1 mL) of propofol. Vomiting is a rare complication, and can be controlled by the administration of antiemetics at the beginning of the mapping phase.
Neurophysiology
The major components of the neurophysiologic protocol are EEG, ECoG, EMG, DES, and MEP techniques. The protocol includes mapping (DES) and monitoring (EEG, ECoG, MEP) procedures.
EEG/ECoG
In the authors’ institution, EEG activity is recorded bilaterally by 4 subdermal needle electrodes, providing 4 bipolar leads. EEG is registered to monitor brain activity when ECoG is not available, at the beginning and the end of surgery, when it is particularly useful for titrating the level of anesthesia. Moreover, it allows assessment of brain activity at a distance from the operating area, such as in the contralateral hemisphere.
ECoG activity is recorded from a cortical region adjacent to the area being stimulated, by means of subdural strip electrodes with 4 to 8 contacts, in a monopolar array, connected to a midfrontal electrode. Cerebral activity is recorded with a bandpass of 1.6 to 320 Hz, and displayed with a sensitivity of 50 to 100 μ/cm for EEG and 200 to 400 μ/cm for ECoG. Continuous electrocorticographic recordings (Comet, Grass Technologies, West Warwick, RI, USA) are used throughout the procedure to monitor the brain basal electrical activity and the level of anesthesia, to define the working current (detection of afterdischarges), and to monitor for the occurrence of afterdischarges, electrical seizures, or clinical seizures during the resection. Because of this, EEG and ECoG recordings should be maintained throughout the operation.
EMG
Continuous multichannel EMG recording (Comet, Grass Technologies) is used throughout the procedure. Several separate muscles (agonist and antagonist muscles) can be monitored in the contralateral or ipsilateral body. Motor responses are collected by pairs of subdermal hooked needle electrodes inserted into the contralateral muscles from face to foot. Each pair of electrodes records 2 different muscles in the same body segment, to sample as many muscles as possible (ie, a flexor and an extensor muscle in the forearm). On average, 16 channels are used for each procedure. The most used setting comprises the face (upper and lower face), neck, arm, forearm, hand, upper leg, and lower leg. A computerized video and image capturing system is continuously coupled with the EMG recordings (Comet, Grass Technologies), to further monitor and register the motor activity. In addition to EMG recordings, motor activity is also evaluated clinically.
MEP
Continuous monitoring of motor function is performed through MEP recording. The “train of 5 technique,” which was introduced for surgery in anesthetized patients, has been described as sensitive for detecting imminent lesions of the motor cortex and the pyramidal pathways. A strip electrode containing 4 to 8 electrodes is placed over the precentral gyrus. In awake patients a single stimulus or a double-pulse stimulus (individual pulse width 0.3–0.5 ms, anodal constant current stimulation, interstimulus interval 4 milliseconds, stimulation intensity close to motor threshold) is usually delivered. The muscle MEPs have to be recorded with needle or (more conveniently in patients who are awake) with surface EMG electrodes. MEP recording is usually alternated with direct cortical and subcortical motor mapping. MEP monitoring is useful because it provides real-time information on the integrity of the motor pathways during the resection of large parts of the tumor not closely related to the functional structures. In addition, MEP provides warnings of impending brain ischemia, caused by critical vessel interruption, mostly in deep temporal or insular regions.
DES
DES for cortical and subcortical mapping is performed with a bipolar hand-held stimulator, with 1-mm electrode tips, 5 mm apart, connected to an Ojemann Cortical Stimulator (Integra Neuroscience, Plainsboro, NJ, USA) or an Osiris stimulator (Inomed, Teningen, Germany). The stimulator delivers biphasic square wave pulses, each phase lasting 1 millisecond, at 60 Hz in trains lasting 1 second for cortical mapping and 1 to 2 seconds for subcortical mapping. Subcortical mapping is alternated back and forth with the resection. Subcortical mapping is performed using the same current threshold applied for cortical mapping.
In mapping performed under general anesthesia, the current intensity ranges from 5 to 15 mA. In patients who are awake, a current intensity ranging from 2 to 8 mA is usually enough to evoke motor responses. In these patients, no electrodes are placed in the mouth, and the activity of the muscles of this region can be checked by monitoring the responses of the patients and by overt inspection. Patients who are awake are asked to relax before and during stimulation, and to assist in the description of induced movements or sensory changes.
The purpose of the mapping procedure is to test motor, language, and cognitive function reliably. At the beginning of the mapping procedure, the initial concern is to define the stimulation parameters. A low frequency of 60 Hz is used to establish the working current. It is advisable to start the procedure with the mapping of motor functions. Once determined, the same intensity of stimulating current is used in most cases throughout the procedure, and for the mapping of cognitive and language functions. Initially, a low-current intensity (2 mA) is used, which is progressively increased until a movement is induced. A stimulus duration of 1 or 2 seconds is usually enough to generate a motor response. At this point, it is good practice to stimulate the areas close to that in which the current induced the movement, to map them, and to check if the current evokes motor responses in these zones. If not, the current intensity may be increased and adjusted to evoke appreciable motor responses. It is also recommended to check with the ECoG if the applied current induces afterdischarges in the nearby brain areas. Only the current that is immediately below the one that induces afterdischarges has to be used for mapping. If afterdischarges are seen, the current should be set at least 0.5 mA less than the previous one. Because only the responses evoked in the absence of afterdischarges are considered to be trustworthy, continuous ECoG recording is used to check the appearance of afterdischarges during the mapping, to maintain the reliability of the test.
For language mapping, the initial test used is counting. The current is usually applied onto the premotor cortex related to the face, and the test aims to check if the current stops the patient counting. This test should be repeated several times, and counting stopped at least 3 times, to be reliable. If not, the current intensity is increased until this is produced. When the current is established, DES is applied to the whole exposed surface of the brain, and the occurrence of afterdischarges checked in the ECoG. The duration of the stimulus is between 3 and 4 seconds. Only the current that is not inducing afterdischarges in the entire stimulated cortex is used for mapping. In afterdischarges, the current intensity is decreased at least by 0.5 mA.
For subcortical mapping, either the same current used for cortical mapping or a current increased by 2 mA is applied, and the stimulus is continuously alternated with the resection. When a response is induced at a subcortical level, the authors perform an intensity-response curve, to assess the maintenance of the response at low current intensity levels. This action can help in estimating the distance between the point of stimulation and the functional tract. During subcortical mapping, ECoG is continuously monitored to look for afterdischarges and seizures, to verify the reliability of the responses.
The resection margin is usually kept at least 5 mm away from functional areas, and may come close to subcortical pathways.
Postoperative protocol
When resection is performed to functional boundaries, most patients develop postoperative deficits. Patients during the postoperative phase are submitted to neuropsychological evaluation (at 3 and 7 days) and to motor rehabilitation, to follow deficit recovery. MR studies, inclusive of volumetric T1, post-Gd T1-weighted images, and volumetric FLAIR images, are also performed. Neuroradiological studies should be performed within 24 to 48 hours of surgery.
Results of the mapping or monitoring procedures
Motor Mapping
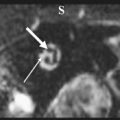
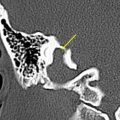
The authors usually map motor responses in patients with tumors located in the rolandic, premotor, or parietal region. Motor mapping is also applied at the cortical and subcortical levels for lesions located in the insula or deep temporal region, in which motor pathways can be encountered during resection. For lesions located in the nondominant hemisphere, the patient is kept under general anesthesia, and the tube positioned through the nose, which allows the placement of a series of electrodes in the inner palate and pharyngeal muscles, and in the tongue, which are useful for detecting responses from these muscles ( Fig. 1 ). For lesions close to or within visuospatial or language areas of pathways, the patient is always awakened during the procedure. In awake and asleep settings, a stimulation duration of 1 or 2 seconds is usually enough to generate a motor response. At cortical stimulation the authors observed different morphologies of EMG responses: cortically evoked responses showed great variations in amplitude, but they always appeared as continuous, tonic bursts of activity, often incrementing during stimulation. The smallest amplitudes were observed in the neck and the shoulder, or in the mouth. Occasionally in patients under general anesthesia who are taking several antiepileptic medications, it might be difficult to evoke cortical motor responses, even after the current intensity has been increased to one that might induce afterdischarges. In these patients, MEP recording can be useful for identifying the location of the motor cortex and to plan the site of incision, allowing continuing resection. During subcortical stimulation, motor responses appeared as focal (few muscles) when the tract was stimulated close to the surface, although they appeared on multiple muscle groups with deep stimulation. Subcortical stimulation evoked tonic bursts and on-off activity (ie, an M-shaped response), peaking at the onset and the end of stimulation. For resection of tumors located in the premotor cortex, the placement of electrodes in the ipsilateral muscles allows responses coming from these segments to be detected during resection. In addition, when resection is approaching the deep portion of the tumor, subcortical stimulation allows the detection of small motor responses without overt muscle activity, which indicates that the resection is close to motor pathways. When these warning responses are identified, resection in this region should be particularly cautious and can proceed until more pronounced motor responses are identified, usually when the tip probe is touching and stimulating the motor pathways. The identification of such pathways is therefore particularly useful for performing an effective resection (see Fig. 1 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree