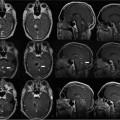
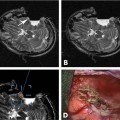
12 Neurosurgical resection of intracranial glioma is the first and most crucial step in treating patients with intrinsic brain tumors: it provides for a correct histologic diagnosis and by debulking the tumor, raised intracranial pressure can be reduced. In the presence of space-occupying low-grade gliomas usually the goal of surgery is to remove the tumor as radically as possible to prevent or to delay recurrence. Previously for high-grade glioma (HGG), both astrocytoma WHO grade III and glioblastoma multiforme (GBM) WHO grade IV, a wait-and-see attitude, biopsy, or removal of a minor part of the tumor have been suggested, followed by radiotherapy with or without chemotherapy. Recently,1 however, it has been posited that surgery would only be useful in cases of massive space-occupying lesions. The surgery would not really affect patient survival because only a portion of the tumor cells would be removed, leaving the rest of the active tumor in the brain. There are times one has to advocate to perform only a brain biopsy to establish a correct diagnosis. Median survival rates for GBM range from 41 to 53 weeks.2–4 Also, infiltration of functionally relevant structures both at the brain surface and in the white matter by the malignant tumor carries the risk of major permanent worsening of neurologic status and thereby quality of life -factors that have been closely related to overall survival following tumor diagnosis and surgery.2,5 This was certainly true with diagnostic imaging based on computed tomography (CT) or even earlier angiography, scintigraphy, and other procedures. Now, with the use of preoperative magnetic resonance imaging (MRI), better delineation of tumor margins can be achieved, as well as the (”microscopic”) extension of tumor beyond the contrast-enhancing tumor margins as seen on contrast-enhanced Tl-weighted images (T1WIs).6,7 Further refinement including MR spectroscopy (MRS) helps to better elucidate tumor biology. Also, the tumor can be correlated to functionally relevant brain areas both by the use of functional MRI (fMRI) and diffusion weighted imaging (DWI), allowing for delineation of the pyramidal tract or intercenter fiber tracts, a process called fiber-tracking.8,9 Over the years, more evidence has accumulated that the most complete tumor removal possible improves a patient’s survival chances. By undergoing radical removal based on MRI criteria, the contrast-enhancing part of the tumor is completely removed as witnessed on an early (maximal 72 hours) postoperative MRI scan, with the understanding that this contrast-enhancing area comprises only part of the tumor and that further malignant tumor spread cannot be ruled out. Jeremic et al10 have shown in a group of 86 patients with GBM that patients treated with more radical surgery had a survival of 56 weeks versus 26 weeks for those who had undergone only biopsy. Also, progression-free survival at 1 year was higher in those radically resected (20% versus 0%). Similarly, Yoshida et al11 showed that besides age, histology, and type of adjuvant therapy, radical resection had a high impact on survival rates. The group of patients who had undergone gross total tumor resection or had experienced a complete response to adjuvant therapy showed survival rates of 42% at 3 years and 24% at 5 years. In a phase II study of multimodal therapy of recurrent malignant gliomas, Rostomily et al12 found in a multivariate analysis that greater surgical debulking and smaller postoperative tumor volumes were associated with prolonged median time to progression (MTP) but not median survival time (MST), which rather was related to a lobar versus deep tumor location. Obwegeser et al13 performed a multivariate analysis in 157 patients with malignant tumors and showed a significant effect of survival after radical macroscopic surgery (p = 0.005), postoperative radiotherapy (p < 0.001), and chemotherapy (p < 0.01). More recently, in our institution a multivariate analysis confirmed these results in a larger cohort and particularly stressed the indication for surgery independent of the patient’s chronologic age;14 however, a Finnish group15 did not show a surgical benefit in older patients. Other groups have demonstrated this beneficial effect of radical removal as well.16,17 Also in a malignant gliomas in childhood study (Childrens Cancer Group Study CCG 945), radical surgical resection (defined as greater than 90% resection as seen on imaging studies – mostly MRI) was shown to be the most powerful predictor of outcome.18 The authors also point to the importance of training of the neurosurgeon in either adult or pediatric neurosurgery. To select GBM patients who might be suitable for radical surgical resection, Shinoda et al19 suggested a topographical grading system based on tumor location, size, and eloquence of adjacent brain as seen on MRI. They found in a multivariate analysis that gross total resection in stage I patients was an independent good prognostic factor. Many techniques have been developed with the aim to support maximal tumor removal. Aggressive tumor removal is usually hindered by the neurosurgeon’s fear of creating a major neurologic and permanent deficit when removing tissue from eloquent areas. Therefore, early in modern neurosurgery and with the refinement of anesthesia and local anaesthetics, awake craniotomy was undertaken with the patient’s cooperation by talking to him or her and making him or her perform voluntary movements during surgery. Details of this technique have been described elsewhere.20,21 Many groups now use this technique on a routine basis. The results reported show that a more radical tumor removal can be achieved using this technique for tumors in eloquent areas. Our group21 recently has reported a 77% complete resection rate for tumor surgery in the motor areas using awake craniotomy compared with only 33% when the patient was operated under general anesthesia. This technique has also been implemented in the setting of high-field intraoperative MRI (iMRI) by our group since early 2006: this is reviewed in Chapter 17. Preoperative imaging provides a good picture of brain anatomy and the tumor; images both from CT and MRI (best in the format of magnetization-prepared rapid gradient echo [MP-RAGE]) can be used for neuronavigation and allow a more-targeted approach to the tumor. However, this method of targeting can be used safely only in skull base surgery and to a minor extent once the dura has been opened. Once the dura is opened, brain shift occurs initially due to release of cerebrospinal fluid (CSF) from the subarachnoid spaces, such as the sylvian fissure and the basal cisterns, and secondarily due to tumor removal.22,23 Best surgical judgment needs to be implemented when proceeding with surgery of a tumor located in the white matter. The location of anatomic structures needs to be taken into consideration, especially the distance of the brain surface from the dura, which depends initially on the position of the patient’s head and may vary considerably from location to location due to inherent brain firmness – “elasticity”- altered by the tumor and the extent of tumor removal. In view of the brain shift it is understandable that only a few groups24 have reported that neuronavigation per se did improve survival of GBM patients (13.4 months versus 10.3 months), whereas our group25 as well as others26 recently had not shown a benefit. However, Wirtz et al24 stated that they had achieved radical resection as defined by postoperative MRI in 31% of all cases, an improvement when compared to 19% in conventional operations. Aminolevulinic acid (ALA) fluorescence has been studied extensively in many phase I to III studies and has been shown to effectively increase the percentage of radical resections in high-grade glioma (HGG) surgery as proven by postoperative MRI scans,27–29 thus prolonging the recurrence-free survival interval. It is now commercially available in Europe as Gliolan (Medac, Wedel, Germany). This technique can safely be applied both in primary and recurrent HGGs. It requires oral application of Gliolan some 3 to 4 hours prior to initiating surgery, and a special microscope lamp producing filtered, violet-blue excitation light for visualizing fluorescence is required. The lamps are available on Zeiss (Carl Zeiss AG, Oberkochen, Germany), Moller-Wedel (Wedel, Germany), and Leica (Sols, Germany) microscopes. The ALA is absorbed by tumor cells and is converted into protoporphyrin IX, which can be detected by utilizing this special light. After regular tumor exposure using white light, the borders of the HGG are delineated by switching to fluorescence mode, and the tumor can be resected using an ultrasound aspirator, guided by the intense red light to depict the highly cell-dense tumor. Vessel damage can also be prevented because arteries and veins show up as pale blue-green structures. Fluorescence is only visible from tissue not immersed in fluids such as blood and CSF. Once the tumor has been removed up to its borders, fluorescence fades away and gives way to a light red (salmon-like) color. The advantage of ALA fluorescence guidance is that it is relatively cheap and highly effective; it guides the surgeon in a rapidly repeatable manner during surgery, and can also be used in recurrent HGG surgery with good accuracy. The problem obviously is that it registers only surface fluorescence: it is impossible to look around corners or underneath the surface. This limitation—together with good surgical judgment not to touch/resect eloquent brain areas—would also explain why using ALA did not give nearly 100% radical resection in the major studies. On the other hand, this technique can also be used on recurrent HGG surgery. Intraoperative ultrasound has been advocated and elaborated by some groups30,31 to intraoperatively enhance surgeons’ vision of tumor borders, and is regularly used in many centers, even coupled to navigation devices. Its advantage again is high flexibility, but its disadvantage is that it is, to a certain degree as all ultrasound devices, a highly subjective method requiring intensive training. With this in mind, it should carry the same radical resection rate as low-field iMRI scanning32 The introduction of MRI into a surgical operating room was what seems today the logical extension of improving preoperative imaging and making it available for intraoperative control in glioma surgery. To be a valuable adjunct for HGG surgery, an iMRI machine needs to have the same scanning properties as commercially available MRI scanners used routinely pre- and postoperatively for all parts of the body. Schwartz33 in Boston, Hall34,35 in Minneapolis, Knauth36 in Heidelberg, and Fahlbusch37 in Erlangen together with various MRI equipment manufacturers were the first to report the implications of iMRI around 1996. Low- and midfield machines were in many sites followed by high-field MRI equipment.38 Many obstacles needed to be overcome such as geometric distortion,39 and the values of various field strengths were repeatedly discussed.40–42 Having overcome initial problems based on the hardware and the magnetic field that prevents the use of regular surgical instruments within it, all groups focused on what patients might benefit the most from iMRI. Those patients requiring radical tumor resection, namely patients with low-grade gliomas (LGG) and pituitary adenomas, were determined to benefit the most from this new applied procedure. Knauth et al36 reported that the extent of glioma resection was more radical (”complete” in 75.6%) using iMRI, in this case a 0.2 tesla (T) machine, as compared to 36.6% in matching patients operated without iMRI. Claus et al43 have reported longer survival rates in a higher percentage of LGG patients operated under iMRI. Only a few reports have been published by the groups mentioned to support the use of iMRI in HGG surgery. Martin et al44 reported on the use of a 1.5 T machine during glioma surgery in 30 patients and found a low frequency of complications while achieving a radiologically complete tumor resection in 80% of the patients. They found it difficult to read the images of contrast-enhancing tumors intra-operatively. This lesson was different from the one learned when operating on LGG; Schneider et al45 reported a good correlation (10 of 12 tumors, the remaining 2 being on the border zone of the LGG) of residual tumor suspected on iMRI using a 0.5 T machine and neuropathologic examination of this area. This group46 also reported a higher resection rate in HGG: a decrease of residual tumor from 29% at first control to 10% at final imaging. Knauth et al47 addressed the problem of delineating a contrast-enhancing lesion (both metastasis and HGG) on the basis of their 0.2 T MRI scanner, comparing the results with those obtained using a standard 1.5 T MRI scanner preoperatively. Based on a “normal” referral basis to a tertiary referral center in a university hospital, we have confirmed that patients harboring HGGs are more common than LGG patients. From September 2005 through May 2009, we operated on 24 astrocytomas WHO grade II, 60 astrocytomas WHO grade III, and on 140 glioblastomas WHO grade IV. In the remainder of this chapter, we will focus on the treatment of HGG patients using iMRI based on our experience over the last 4 years. Details of the operating room setup have been published in previous articles from our group48,49 and are also presented in chapters of this book. We have adapted the iMRI setting from the Minneapolis group,34,35 using a short-bore Philips Intera 1.5 T MRI scanner (Philips Healthcare, Andover, MA), but in a dedicated MRI operating room (OR) that was designed specifically for this purpose when we constructed a new building with four ORs for our department. From September 2005 until May 2009, we have treated 426 patients in this MRI-OR. Patients presented with a variety of disease entities: mostly HGG (n = 200), followed by pituitary adenomas (n = 64) and LGG (n = 24). Meningiomas, metastases, and cavernomas were rarely seen, and spinal tumors were rarer still. This setting is also used for preoperative stereotactic imaging for deep brain stimulation (DBS).
Intraoperative MRI Scanning in High-Grade Gliomas
Awake Craniotomy
Neuronavigation
Aminolevulinic Acid Fluorescence
Intraoperative Ultrasound
Intraoperative Magnetic Resonance Imaging
Technique
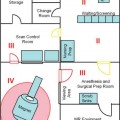
Operating Room Setting
Patient Positioning and Safety
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree