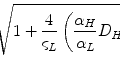(1)
Centre for Neurosciences, Ninewells Hospital and Medical School, Dundee, DD1 9SY, Scotland, UK
Abstract
Malignant brain tumours (MBTs) comprise primary malignant tumours (PMTs) and metastatic tumours (METs). MBTs are responsible for 3 % of all cancer deaths worldwide and are the second most common cause of cancer death in young people. They carry a very dismal prognosis, typically representing a death sentence that may be deferred by up to merely 36 weeks inspite of treatment (Obwegeser et al. 1995; Eljamel 2004). PMTs represent 40 % of all primary brain tumours, and glioblastoma multiforme (GBM) is the commonest type (Stupp 2007). Although progress has been made in the pathophysiology of GBMs, overall survival remains very poor and individual prediction of clinical outcome remains an elusive goal. Despite extensive clinical trials, the median survival of patients with GBM is still 12–14 months, with fewer than 26 % surviving for 2 years. In a series of 279 patients, only five (1.8 %) survived for 3 years (Lamborn et al. 2004). Multimodality therapeutic approaches would be necessary to improve this dismal outcome.
13.1 Introduction
Malignant brain tumours (MBTs) comprise primary malignant tumours (PMTs) and metastatic tumours (METs). MBTs are responsible for 3 % of all cancer deaths worldwide and are the second most common cause of cancer death in young people. They carry a very dismal prognosis, typically representing a death sentence that may be deferred by up to merely 36 weeks inspite of treatment (Obwegeser et al. 1995; Eljamel 2004). PMTs represent 40 % of all primary brain tumours, and glioblastoma multiforme (GBM) is the commonest type (Stupp 2007). Although progress has been made in the pathophysiology of GBMs, overall survival remains very poor and individual prediction of clinical outcome remains an elusive goal. Despite extensive clinical trials, the median survival of patients with GBM is still 12–14 months, with fewer than 26 % surviving for 2 years. In a series of 279 patients, only five (1.8 %) survived for 3 years (Lamborn et al. 2004). Multimodality therapeutic approaches would be necessary to improve this dismal outcome.
The joint tumour section of the American Association of Neurological Surgeons/Congress of Neurological Surgeons produced guidelines for the management of GBMs (Scott et al. 1998). The guidelines recommend maximum safe surgical resection, followed by 60 Gy of radiotherapy to the enhancing lesion, encompassing a 2-cm cuff around the lesion. The guidelines also recommend concurrent chemoradiation using Temozolomide and BCNU (Gliadel) in those who undergo craniotomy. Temozolomide offers a 2-year survival rate of merely 26 %, with median survival of 14.6 months and progression-free survival of 7.2 months (Olsen and Ryken 2008), while Gliadel offers survival of 13.9 months (Olsen and Ryken 2008). The poor outcome of these tumours is due to local invasion and local relapse. Eighty percent recur locally within 2 cm of the resection margin and patients often succumb to local recurrence, emphasising that a more aggressive local therapy is still awaited. However, complete radical surgical excision is hindered by the significant amount of tumour that is invisible to the surgeon even with the aid of the surgical microscope; indeed, its complete identification at surgery is an impossible task. Most of these tumours have invaded the brain widely by the time they manifest clinically, making a wider excision margin out of the question.
On the other side of the coin, METs are much more common (Vecht et al. 1990). They have an incidence of 12/100,000 per year and 15–40 % of systemic cancers metastasise to brain. The most frequent sources are lung tumours (40–50 %), breast cancers (15–25 %) and melanoma (5–20 %) (Vecht et al. 1990). The frequency of METs appears to be rising as a result of improved brain imaging and more effective treatment of primary cancers (Klos and O’Neill 2004; Chang et al. 2007). Common clinical features of METs are similar to those of PMTs, i.e. headache, neurological deficit and seizures.
If METs remain untreated, they are rapidly fatal. Surgery is indicated for solitary metastases in patients with a good performance status, and when the primary cancer is under control (Mehta et al. 2003). However, eradication of METs is not always possible owing to inability to resect the tumour en masse with a cuff of normal brain tissue, and adjuvant therapies are often used, e.g. whole brain radiotherapy, stereotactic radiosurgery, interstitial radiotherapy and chemotherapy.
Overall, therefore, aggressive local multimodality therapy holds the key in the successful treatment of MBTs, and the Photon Radiosurgery System (PRS) provides a means to deliver local interstitial radiotherapy at the same time as surgery.
13.2 Use of PRS in the Treatment of Brain Tumours
13.2.1 Rationale
The PRS was initially designed for use in MBTs using the Cosman-Roberts-Wells (CRW) stereotactic frame. That is why the PRS probe is 3 mm wide and 160 mm long: this permits placement of the active tip in the centre of the CRW arc. The unit weighs 3.6 lb. The shape and diameter of the probe neck is designed to fit the CRW frame (Figs. 13.1 and 13.2).

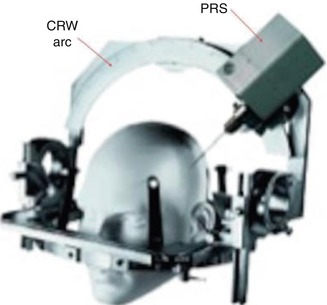

Fig. 13.1
Photograph of the PRS designed to fit the CRW frame

Fig. 13.2
Schematic drawing demonstrating the PRS in the CRW frame
The basic principles of the design were intended to enable neurosurgeons to obtain a biopsy using the CRW frame followed by interstitial stereotactic intraoperative radiosurgery (IORS) at the same sitting. After the early neurosurgical experience, it was realised that a wider applicator was essential to enable PRS use during open surgical resection (Fig. 13.3).
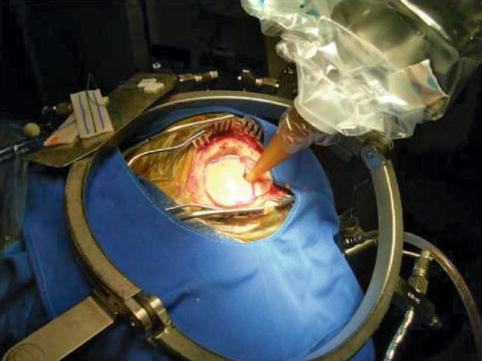

Fig. 13.3
Intraoperative photograph of the PRS with an applicator in place. Note that the bone flap was replaced to reduce radiation
13.2.2 Clinical Experience
Overall, 151 patients with MBTs have been treated using the PRS, including 14 children. The adult population has included 61 PMTs and 64 METs with a female to male ratio of 1.1 and a mean age of 56 years. Of the 64 METs, 51 have been solitary; the mean dose has been 15 Gy and the mean treatment time, 20 min (Fig. 13.4). The side-effects in these patients have been seizures in 1.8 %, cerebral oedema in 2.4 % and unrelated complications in 0.8 %, with zero mortality and zero neurological morbidity. The findings of particular studies are discussed in more detail below, under “Results”.
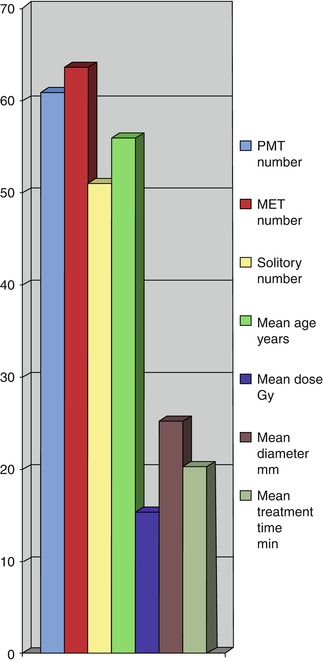

Fig. 13.4
Clinical features of patients with brain tumours treated by the PRS
13.2.3 Dosimetry
The PRS produces a radiation field similar to that of a conventional high-dose interstitial brachytherapy source, but with controllable intensity and peak energies (Dinsmore et al. 1996). The X-ray beam behaves essentially as a point isotropic source (current of 40 Amp and voltage of 30–50 kV), giving 15 Gy/min at a distance of 10 mm. Because the photons produced by the PRS are low energy, the X-rays produced are attenuated rapidly within the brain and a dose decline rate of 1/r 3 is obtained, rather than the 1/r 2 seen for standard higher energy radioactive isotopes. The resultant 30 % dose reduction per millimetre of brain tissue creates an extremely steep dose fall-off, and background exposure to personnel more than 2 m from the probe upon insertion is approximately 0.05–0.1 mSv/h. Therefore no special shielding of the patient or healthcare personnel is required (Beatty et al. 1996; McDermott et al. 1996) (Fig. 13.5).