Fig. 7.1
Angiographic underestimation of disease. Although angiogram (top) shows only minor luminal irregularities, two sites in the left anterior descending artery (arrows) show major atherosclerosis (below). The entire vessel was diffusely diseased (Reproduced with permission from Nissen andYock [1])
Equipment and Technique (Fig. 7.2)
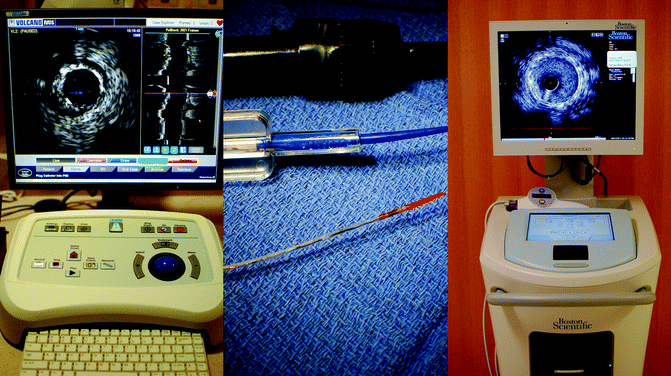
Fig. 7.2
(a, b) IVUS imaging systems. Left panel: The Volcano S5i™ imaging system (Volcano Corporation, San Diego, CA). Center panel: The Volcano Eagle Eye® Platinum IVUS catheter (Volcano Corporation, San Diego, CA). Right panel: The Boston Scientific iLab® imaging system (Natick, MA)
IVUS catheters provide high-resolution cross-sectional images of the coronary arteries through the use of high frequency ultrasound.
Typical IVUS ultrasound imaging is performed at 20–40 mHz, allowing a resolution of approximately 150–200 μm.
A small transducer is mounted on the end of a flexible catheter which can reach distal segments of most coronary arteries. Current IVUS catheters range in size from 2.6 to 3.5 French (0.87–1.17 mm) and are inserted through 6-French guiding catheters.
Two types of IVUS transducers (Fig. 7.3):
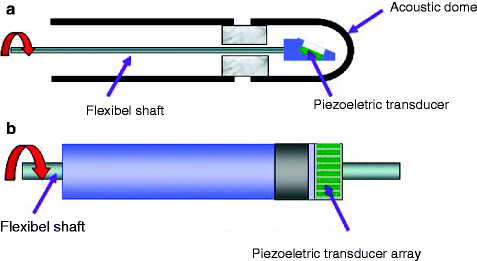
Fig. 7.3
(a, b) Two types of intravascular ultrasound (IVUS) transducers: mechanical rotating (a) transducer or phased array transducer (b)
Mechanical catheters have a single rotating ultrasound crystal at the catheter tip which rotates at approx 1,800 rpm to obtain cross-sectional view of the entire vessel.
Solid state or phased array catheters have multiple piezoelectric transducers mounted around the circumference of the catheter tip. Each of these individual transducers is activated sequentially to produce a rotating ultrasound beam.
Motorized catheter pullback is often used for imaging acquisition, typically at 0.5 mm/s.
Safety
IVUS imaging is safe with a low rate of complications.
In 7,085 IVUS studies, major complications (dissection, thrombosis, ventricular fibrillation, and refractory spasm) occurred in 0.14 %. Vasospasm occurred in 3 % [2].
Known complications and relative contraindications are listed in Table 7.1.
Table 7.1
Relative contraindications and complications of IVUS
Relative contraindications to IVUS
Bacteremia or sepsis
Major coagulation system abnormalities
Patients disqualified for CABG surgery
Patients disqualified for percutaneous coronary intervention
Severe hemodynamic instability or shock
Patients diagnosed with coronary artery spasm
Total occulsion
Complications of IVUS
Arterial dissection or perforation
Total occlusion
Death
Abrupt vessel closure
Acute myocardial infarction
Ventricular fibrillation
Unstable angina
Air embolism
Coronary Artery Anatomy
The three layers of the artery (intima, media, adventitia) can often be visualized by IVUS (Fig. 7.4):
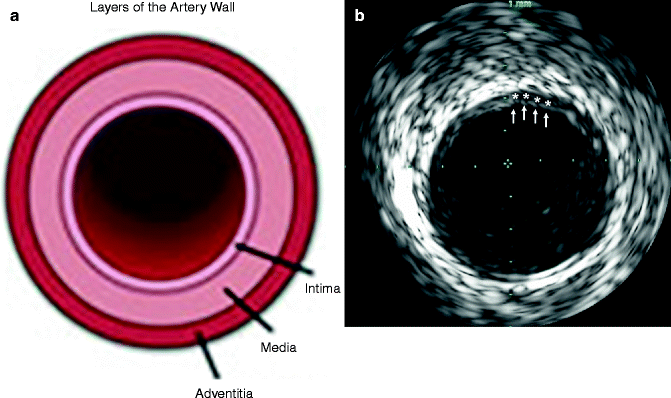
Fig. 7.4
(a) Diagram depicting, in cross-section, the layers (intima, media, adventitia) of the arterial wall. (b) Corresponding intravascular ultrasound (IVUS) imaging demonstrating the blood-intima interface (arrows) and evidence of mild atherosclerotic plaque evidenced by thickening of the intima-media layer
The intima is the innermost layer and normally is only one or two cell layers thick. The intima can enlarge significantly with plaque deposition. The intima appears on IVUS as an echobright layer.
The media surrounds the intima and is a layer of smooth muscle cells. This normally appears as a dark stripe on IVUS.
The adventitia surrounds the media and is composed of fibrous connective tissue. This appears as multiple bright echoes.
Atherosclerotic disease results in thickening of the intimal layer with plaque deposition:
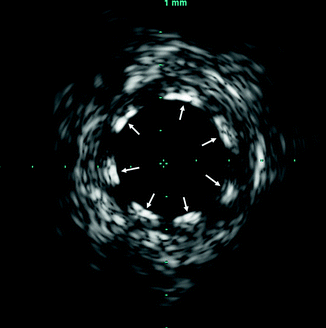
Eccentric plaque deposition is readily apparent due to the tomographic view of the artery with IVUS (Fig. 7.5).
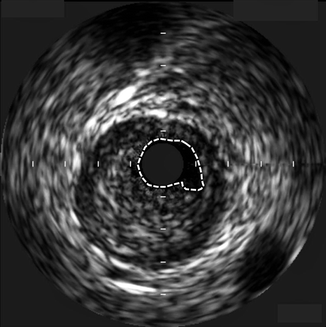
Fig. 7.5
Large, eccentric, stenotic coronary plaque visualized by intravascular ultrasound (IVUS). The dotted lines represent the tracing of the residual for calculation of the minimum luminal area at this point. The circle in the center of the image is the IVUS catheter
Calcified plaque reflects ultrasound waves and results in a bright hyperechoic lesion with IVUS. Because of the lack of penetration of ultrasound waves, an acoustic shadow will be apparent behind calcific lesions (Fig. 7.6).
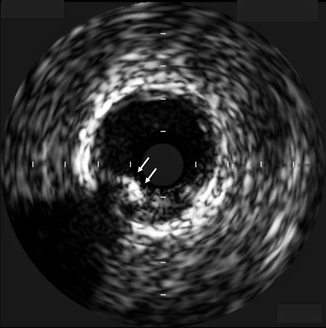
Fig. 7.6
Intravascular ultrasound (IVUS) image demonstrating a partially calcified plaque (arrows) that appears echo-bright. Note the acoustic shadowing beyond the calcified area
Measurements [3] (Fig. 7.7)
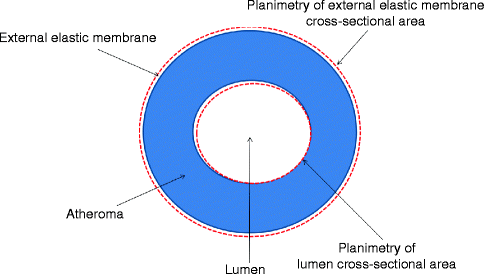
Fig. 7.7
Schematic of tomographic image of coronary artery with atheroma (blue) demonstrating various planimetered measurements (red lines) used in calculations of external elastic membrane, lumen, and atheroma cross-sectional areas. From these areas, the percent atheroma burden and lesion area stenosis can be calculated
Lumen cross-sectional area (CSA) is quantified by planimetry of the intimal leading edge.
The external elastic membrane (EEM) CSA is the outer vessel border. It is measured by tracing the media-adventitia border.
The plaque plus media (or atheroma) CSA is the difference between the EEM CSA and the lumen CSA.
The percent plaque burden is calculated by the following formula: (atheroma CSA/EEM CSA) × 100.
The lumen area stenosis differs from the percent plaque burden by comparing the minimal lumen area of the stenosis to a reference lumen either proximal or distal to the stenosis. The area stenosis can be calculated by the following formula: (reference lumen CSA – minimum lumen CSA)/reference lumen CSA.
Uses for IVUS
Intermediate Coronary Lesions
Left main coronary artery lesions can be difficult to assess due to such factors as ostial location or overlapping vessels:
There is no consensus regarding the best IVUS-derived measurement for significant left main disease.
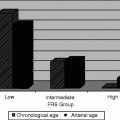
Minimal lumen diameter of greater than 3.0 mm of the left main coronary artery was found in a study of 122 patients to have low risk of adverse cardiovascular events after 1 year [4].
A study of 121 patients with normal or minimally diseased left main coronary arteries determined the lower range of normal minimal lumen area to be 7.5 mm2. Two hundred and fourteen consecutive patients with indeterminate left main lesions were then recommended for surgical revascularization if the minimal lumen area was less than 7.5 mm2. In 3 years of follow-up, there was no significant difference in major adverse cardiac events (target vessel revascularization, myocardial infarction, all-cause death) among those patients with MLA < 7.5 mm2 who underwent surgery and those with MLA > 7.5 mm2 in whom surgery was deferred (20.8 % vs. 11.6 %, P = 0.28). Event rates were 50.1 % in those with MLA < 7.5 mm2 who did not undergo surgery [5] (Table 7.2).
Table 7.2
Long-term follow-up
MLA ≤ 7.5 mm2
MLA ≥ 7.5 mm2
A (Revascularization)
B (Deferral)
C (Revascularization)
D (Deferral)
n
71
12
17
114
n with follow-up
61 (85.9)
11 (91.7)
16 (94.1)
97 (85.1)
Follow-up period (years)
3.2 ± 2.2
3.0 ± 2.2
3.8 ± 1.7
3.6 ± 2.1*
Range (years)
(0–7.7)
(0.1–7.1)
(1.1–7.3)
(0.1–8.4)
Target vessel revascularization
1
4
2
8
Myocardial infarction
7
1
0
2
All-cause death
9
2
1
11
Three-year freedom from MACE (%)
79.2
49.9
93.8
88.4*,†
IVUS was compared to fractional flow reserve (FFR) for assessment of intermediate left main coronary artery stenoses. A minimal lumen area of less than or equal to 5.9 mm2 or minimal lumen diameter of less than or equal to 2.8 mm were found to correlate best with a hemodynamically significant lesion by FFR [6, 7].
Several small studies of patients with intermediate non-left main coronary artery stenoses have compared IVUS with various physiologic tests such as myocardial perfusion SPECT imaging [8], coronary flow reserve [9], and FFR [10, 11]:
Various IVUS parameters have been evaluated using receiver operating characteristic curves and have demonstrated modest correlation with these physiologic tests to determine hemodynamic significance of stenoses.
Minimal lumen area (MLA) of <4.0 mm2, area stenosis of >70 %, and minimal lumen diameter of ≤1.8 mm have demonstrated excellent sensitivity and fair specificity at detecting an abnormal FFR value in a study of 53 lesions in 43 patients. The combined assessment of an area stenosis >70 % and minimal lumen diameter of <1.8 mm increased the diagnostic accuracy compared to FFR with a sensitivity of 100 % and specificity of 76 % [9].
A similar study of 51 lesions in 42 patients established IVUS parameters of a minimal lumen area of <3.0 mm2 and area stenosis of >60 % as correlating best with FFR. This same study found the combined findings of area stenosis >60 % and MLA < 3.0 mm2 to have a sensitivity of 100 % and specificity of 90 % when compared to FFR [12].
Guiding Coronary Intervention
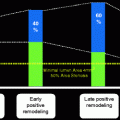
By providing precise detail regarding the size of vessels, extent of atherosclerotic plaque, and composition of plaque, IVUS is often used to guide percutaneous coronary interventions (PCI) (Figs. 7.8 and 7.9). IVUS can also determine adequate stent apposition. Malapposition of drug-eluting stents has been determined to be a risk factor for subsequent stent thrombosis (Figs. 7.10 and 7.11).
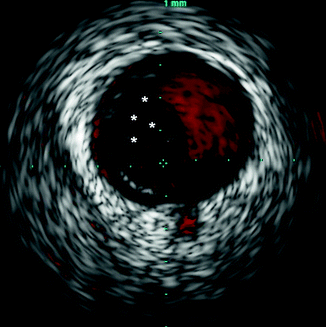
Fig. 7.8
Intravascular ultrasound (IVUS) image with color imaging (ChromaFlo®, Volcano Corporation, Rancho Cordova, CA) highlighting the blood within the coronary lumen in red. The area with no color flow (asterisks) represents likely intraluminal thrombus
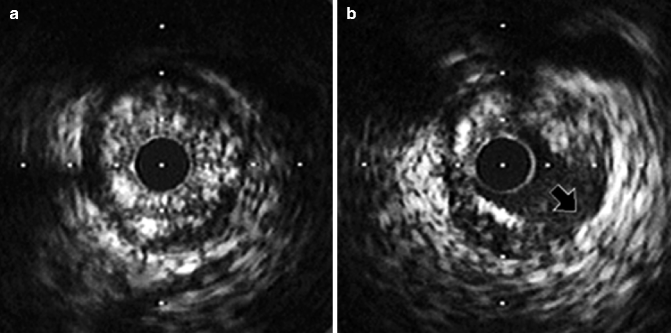
Fig. 7.9
(a, b) Dissection after balloon angioplasty. The pre-interventional image (a) shows circumferential plaque. (b) Shows a dissection extending to level of media (arrow) (Reproduced with permission from Nissen and Yock [1])