Fluorescent probe
Stock conc.
Store at (°C)
IV injection (mg/mL)
Intra-ductal
Intra-stromal
Hoechst 33342
10 mg/mL
4
0.02
–
–
FM1-43
20 mM
−20
–
200 μM
20 μM
Texas-Red DHPE
20 mM
−20
–
100 μM
20 μM
Cascade Blue 10 kDa dextran
25 mg/mL
4
–
1 mg/mL
50 μg/mL
Alexa 488 10 kDa dextran
10 mg/mL
4
–
1 mg/mL
50 μg/mL
Texas-Red 10 kDa dextran
25 mg/mL
4
–
1 mg/mL
50 μg/mL
Alexa 647 10 kDa dextran
10 mg/mL
4
–
1 mg/mL
50 μg/mL
Oregon G. 488 70 kDa dextran
10 mg/mL
4
0.4
–
50 μg/mL
Texas-Red 70 kDa dextran
25 mg/mL
4
0.4
–
50 μg/mL
FITC 500 kDa dextran
10 mg/mL
4
1
–
–
FITC 2,000 kDa dextran
10 mg/mL
4
2
–
–
Tetramethylrhodamine 2,000 kDa dextran
10 mg/mL
4
–
–
2.3 In Vivo Gene Delivery to Submandibular Salivary Gland
1.
SZX7 Stereo microscope mounted on an adjustable arm (Olympus America, Center Valley, PA).
2.
Custom-made stereotactic device for salivary duct cannulation (see Fig. 1a–c).
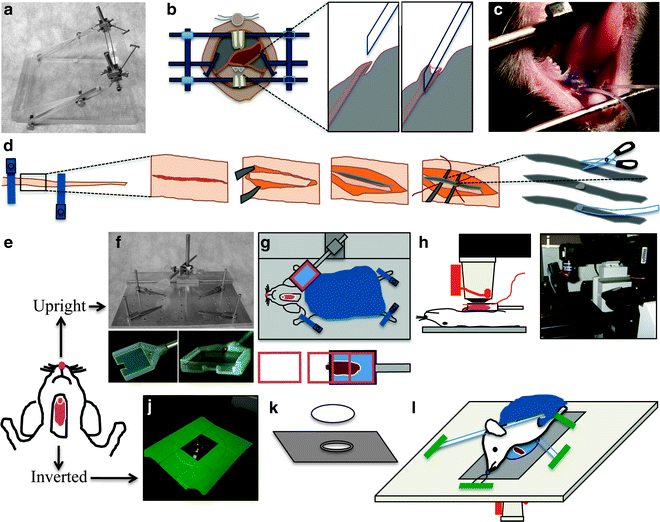

Fig. 1.
Animal preparation for intravital microscopy. (a–c) Cannulation of the Wharton’s duct. (a) Custom-made stereotactic device for cannulation. (b) Diagram showing the Wharton’s duct cannulation procedure. (c) Picture of an anesthetized rat, immobilized on the stereotactic device. Two PE-5 cannulae were inserted into the ducts. (d) Diagram of tail artery catheterization procedure. The tail artery is isolated, an incision is made, and PE-5 tubing is inserted. (e) Diagram showing the rat after SGs externalization surgery. The animal can be positioned for imaging either for upright or inverted configuration. (f–i) Setup for the upright configuration. (f) Custom-made animal stabilization stage with salivary gland (SG) holder. Lower panels show the details of SG holder. (g) Diagram showing the positioning of the animal for the upright configuration. Lower panel shows the insertion of the coverglass. (h) Diagram showing the animal in a side view on the stage. The objective equipped with a heater is brought on top of the coverslip covering the SG. (i) Picture showing the microscope in the upright configuration. Objective and heater are mounted onto an objective inverter. (j–l) Setup for the inverted configuration. ( j ) A round 40 mm cover slip secured to a 35 mm inverted dish holder stage insert is pictured. Plan-Apo 60x water-immersion objective is visible. (k) Diagram showing the 35 mm dish holder stage insert and a coverslip. (l) Diagram showing the positioning of the animal for the inverted configuration. The animal is stabilized by a suture holding the incisors and several bars holding down the neck and the thorax areas. These stabilization devices are held in place by masking tape.
3.
Polyethylene PE-5 cannula (Strategic Applications, Libertyville, IL) (see Note 4).
4.
Histoacryl tissue adhesive (TissueSeal, Ann Arbor, MI).
5.
1 mL Plastic syringes.
6.
30 G 1/2 in. needle (Exelint, Los Angeles, CA).
7.
Plasmid DNA purified from maxi-prep kit (Qiagen, Valencia, CA).
8.
In vivo-jet PEI polyethyleneimine (PEI) (Polyplus Transfection, New York, NY).
2.4 Animal Holders and Positioning Devices
1.
Custom-made immobilization stage (Fig. 1f). Alternatively, the stage can be assembled from components that are commercially available (Thorlabs, Newton, NJ): Aluminum Breadboard, 8″ × 8″ × 1/2″, ¼–20 Threaded (catalog # MB8), Mini-Series Swivel Post Clamp (catalog # MSWC), Cage Assembly Rods, 4″ Long, ∅6 mm (catalog # ER4), Thread Adapter, 1/4″-20 to #4-40 (catalog # MSA25) (see Note 5).
3.
22 mm × 22 mm glass coverslips, # 1.5 and # 0.
4.
Stage insert for 35 mm dishes to accommodate a 40 mm glass coverslip (Olympus America, Center Valley, PA) (Fig. 1j–l) (see Note 5).
5.
Glass 40 mm round coverslips, # 1.5 (Bioptechs, Butler, PA).
6.
Custom-made salivary gland stabilizers for inverted configuration.
7.
Objective warmer system (Bioptechs, Butler, PA).
8.
Gauze sponges 4″ × 4″ (Tyco Healthcare, Gosport, UK) to be used as blankets.
9.
Disposable Foot Warmers (Heat Factory, Vista, CA).
2.5 Microscope
1.
IX81 inverted confocal microscope, equipped with a Fluoview-1000 scanning unit (Olympus America, Center Valley, PA) modified for multiphoton microscopy, as described in (8) (see Note 6).
2.
Plan-Apo 60x, 1.2 NA, water-immersion objective (Olympus America, Center Valley, PA) (see Note 7).
3 Methods
Subcellular structures can be labeled using different strategies, such as systemic delivery of fluorescent probes, transfection of fluorescently labeled proteins, or a combination of both. A flow chart of the procedures described hereafter is provided in Fig. 2. Salivary glands can be imaged using either an upright or an inverted microscope. The choice depends on the characteristics of the available microscope. The upright configuration requires the use of custom-made holders, whereas the inverted configuration requires particular care in immobilizing the animal.
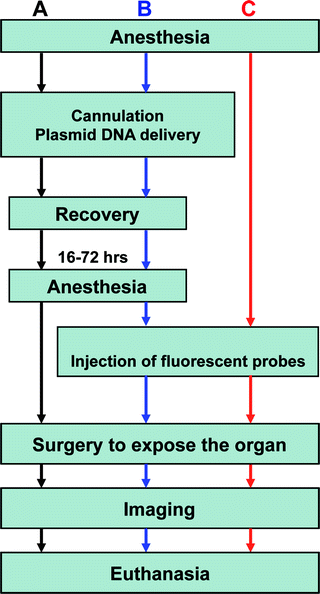

Fig. 2.
Flowchart of common experimental procedures for intravital microscopy. (a) Workflow for imaging only the transfected cells in vivo. (b) Procedures for imaging a combination of expressed fluorescent proteins and injected fluorescent probes. (c) Workflow for visualizing fluorescent probes introduced into the SG tissue. Note that the injection of the fluorescent probes can be performed while imaging.
3.1 Animals and Anesthesia
1.
Rats are housed in a controlled environment in the local animal facility and 1 week prior to the experiments are moved and housed in the lab to acclimate (9). Water and food are provided ad libitum.
2.
Weigh the animal prior to anesthesia.
3.
Gently place the animal into the vaporizer chamber, and turn on the oxygen flow (800–900 mmHg). Set the Isofluorane level to 3% to proceed with the anesthesia induction (see Note 8).
4.
Once the animal is unconscious, inject a mixture of 100 mg/kg ketamine and 20 mg/kg xylazine (see Note 1) into the peritoneal cavity (25-gauge needle).
5.
Observe the animal for signs of wakefulness and inject more anesthetic mixture as needed (75% of the initial dose within 45 min after the first injection and 50% every hour thereafter (see Note 9)).
6.
Apply eye lubricant to prevent eye dryness.
7.
Place the animal under the heat lamp or on a Foot Warmer pad.
8.
Monitor the body temperature of the animals with the MicroTherma 2T Thermometer equipped with a RET-3 rectal temperature probe (see Note 10).
3.2 In Vivo Gene Delivery to Submandibular Salivary Gland
1.
Secure the anesthetized animal into the stereotactic device with the mandibles wide open and the cheeks extended to the sides (Fig. 1b). The tongue should be folded towards the back of the mouth to expose the ductal orifices without obstructing the airways.
2.
Incline the stereotactic device at approximately 45° and adjust the stereomicroscope to visualize the area below the tongue.
3.
Focus on the two orifices of the Wharton’s ducts. They will appear as two small flaps of tissue in the center of the area under the tongue (Fig. 1b).
4.
Use bent sharp #7 tweezers to grab the PE-5 cannula close to the tip (see Note 4). Gently push the orifice with the tip of the cannula until it gets inserted into the duct (see Fig. 1b and Note 11)
5.
Once the Wharton’s duct is cannulated, apply a small drop of histoacryl tissue glue to the orifice, and let it dry.
6.
Prepare the transfection mixture according to the PEI manufacturer’s instructions. Typically, efficient transfection is achieved by using 12–24 μg of DNA/gland in a final volume of 100 μL (see Note 12).
7.
Aspirate the transfection mixture with a syringe (30-gauge needle) and make sure that no air bubbles are released when injecting the fluid.
8.
Carefully connect the needle to the cannula without piercing the tubing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree