1
Introduction
Candice Johnstone
Cancer is a global problem with an estimated 14.1 million new cancer cases worldwide, 32.6 million people living with cancer, and 8.2 million cancer deaths in 2012.1 Thus, the need for good palliative care (PC) is also increasing globally as patients with advanced cancer require a coordinated multidisciplinary PC effort.
There are two fundamental categories of palliative caregivers: (a) generalist and (b) specialist. Every person who provides health care to patients with cancer or other serious illnesses should be considered a generalist and should have a comfort level with the fundamentals of PC. These fundamentals include talking with a patient to understand their goals, devising a treatment or supportive care plan consistent with those goals, management of commonly encountered symptoms and discussions about life expectancy and care at the end of life (EOL). Specialists are clinicians that have completed a PC fellowship or have additional certification in PC. They help manage refractory symptoms, navigate difficult or complex family dynamics, and help patients evaluate existential distress.2
PC aims to reduce pain and suffering, facilitate discussions of goals of care and death with dignity, promote quality of life, and support patients, their families, and their caregivers. The assessment includes pain and symptom assessment as well as an assessment of the social and spiritual context. Patients whose spirituality is supported by the medical team have experienced better outcomes and quality-of-life.3–5 One commonly cited barrier noted by medical practitioners is the lack of training about how to provide such care.6 PC providers can learn to incorporate discussions of religion and spirituality into the care that they deliver to support the spiritual needs of the patients they care for.7 Simple acronyms, such as FICA,7 help facilitate these conversations.
F represents faith, belief or meaning, I stands for importance and influence, C for community, and A represents address or action in care. This tool allows the health care team to determine if there is a particular faith or set of beliefs that provides meaning for a patient, assess how this faith or spirituality affects that person’s life, their coping skills and decision making. This discussion can then be incorporated into subsequent care (Table 1.1).
TABLE 1.1 FICA Spiritual History Tool
FICA Spiritual History Tool | ||
F | Faith, belief or meaning | Do you have faith? What gives your life meaning? |
I | Importance | Do these beliefs help you cope or make decisions? |
C | Community | Do you belong to a community? |
A | Address or action | Health care team incorporates this knowledge |
Prognostication and accurate estimates of life expectancy are essential for goal setting and appropriate treatment selection. Increasingly, quality metrics surrounding the appropriate use of aggressive therapies are being developed. Avoidance of radiation therapy (RT), especially long-courses of RT, at the very EOL is important given the time course of the palliative effect of radiation. Whenever possible, the shortest course of RT likely to have the desired effect should be employed. This maximizes patient comfort and convenience and minimizes cost and toxicity.8,9
RT plays a key role in the palliation of physical symptoms caused by advanced cancer and serves as an adjunct to systemic analgesics, chemotherapy, interventional procedures, and supportive care. As such, radiation oncologists are essential members of a multidisciplinary PC team.
In a world of evidence-based practice and cost consciousness, physicians will increasingly utilize single fraction and short-course external beam palliative radiation courses. These approaches are critical parts of the practicing physician’s knowledge base as they are generally equally efficacious, more convenient for patients, and more cost-effective.
Twenty-five randomized trials have demonstrated the equivalence of single fraction palliative radiation to longer, more expensive courses of therapy in the treatment of uncomplicated bone metastases, yet this approach is infrequently used. Single fraction RT has been hailed as a quality metric and singled out as part of the “Choosing Wisely” campaign. Similarly, in other clinical situations, there is a body of literature demonstrating equivalence of shorter courses of therapy. These approaches are more convenient for patients, many of whom have discomfort during transport.
As our health care economy moves more toward value-based care, more physicians will likely prescribe shorter courses of palliative radiation. In addition, if physicians or hospitals are reimbursed for an episode of care rather than a fee for service they will look to decrease the cost of a course of RT and utilize more cost-effective, shorter radiation regimens.
Barriers to implementation of short course and single fraction therapies include a lack of knowledge about the evidence supporting these approaches, the lack of familiarity with evaluating plans utilizing higher dose per fraction RT, and early experience using high-dose per fraction for curative therapies where the long-term toxicity proved excessive. Many physicians have never prescribed hypofractionated palliative radiation as economic incentives in the United States have long favored multifraction radiation regimens. Thus, many trainees have never been exposed to the practice. In addition, in the early days of RT, higher dose per fraction of radiation was associated with significant late complications. This is generally due to the use of large fractions in patients treated with curative intent without adjustment of the total dose of radiation to minimize late toxicity. Thus, patient selection and life expectancy are critical to the use of short course or hypofractionated palliative RT.
LIFE EXPECTANCY AND PROGNOSTICATION
Questions about life expectancy and the quality of that remaining life are extremely important to patients with metastatic cancer. Physicians and other health care providers often provide overly optimistic estimates of survival; these estimates often differ from actual survival by 3 months or more.10 Accurate estimates of life expectancy help patients set appropriate goals, avoid futile treatments, and choose supportive care or treatments that will be effective within their remaining lifetimes.
Several themes emerge from the existent prognostication literature. Clinical predictions generally overestimate survival, but improve with repeated encounters. The patient’s Karnofsky performance score is the strongest predictor of life expectancy, but other clinical factors help predict actual estimates of survival. Very poor prognostic factors include the presence of the symptom cluster known as the terminal syndrome (dyspnea, dysphagia, dry mouth, anorexia, and weight loss) or the presence of cognitive failure or confusion.11
Several tools were developed in the palliative RT setting and are detailed in Chapter 2 and Appendix B details tools developed in other settings. Some are easier to utilize and are more generalizable than others. The best use of these tools may be in deciding which patients may not live long enough to see the benefit of a particular treatment, since RT typically takes several days to a few weeks for its palliative effect to be seen. Patients with an extremely short life expectancy with hemoptysis and spinal cord compression may still benefit from RT as hemostasis can occur within 24 to 48 hours and the functional decline associated with spinal cord compression may significantly impair quality of life. Administration of chemotherapy within the last month of life has been promulgated as a metric of overutilization of health care.9,10 Similar metrics may follow for RT.12
PALLIATIVE RT
External beam RT is a key component of palliative cancer care. It is useful to treat pain due to osseous metastasis or local tumor invasion, bleeding, obstruction, dyspnea, or cough, and functional impairment due to brain metastasis or impingement of nerve roots or the spinal cord.
Key in the utilization of RT is the selection of the shortest fractionation regimen that is effective to maximize patient and caregiver convenience and minimize toxicity and cost.13–16 See Appendix A for fractionation schemes based on life expectancy.
Though many believe that longer courses of RT in the treatment of bone metastasis have a more durable effect, there is no data to support this belief. In the Radiation Therapy Oncology Group (RTOG) 97–14 randomized trial of fractionation, only patients with a long expected survival were enrolled. There was no difference in efficacy between 8 Gy in a single fraction and 30 Gy in 10 fractions.13 Similarly, in an analysis of those patients who survived more than 52 weeks in the Dutch Bone Metastasis Study, there was no difference in response rate, time to response, duration of response, and time to progression of pain.17 Randomized trials have confirmed the equivalence of short courses of RT in lung cancer18–20 and bladder cancer,21 and hypofractionated radiation regimens have been successfully used to treat gynecologic, gastrointestinal, and head and neck malignancies.15
This handbook aims to familiarize the reader with the literature supporting the use of palliative RT in various settings, and to provide a detailed guide to radiation planning and choice of fractionation with illustrative pictures and case presentations. There will be an emphasis on short course or hypofractionated approaches when appropriate.
Efficacy of palliative RT
RT provides effective pain relief in the vast majority of patients with few side effects. RT can decrease the need for systemic analgesia and can be extremely useful in patients who are intolerant of opioid analgesics.22
The time course for pain relief after the delivery of RT is variable and is generally not immediate. Some patients experience relief within a few days or a week of treatment but the full palliative benefit may not be evident for 4 to 6 weeks. Patients with a life expectancy less than 30 days may not benefit from RT and alternative palliative therapies should be explored. Since RT requires immobilization during treatment delivery, patients must have adequate pain relief prior to the initiation of therapy. The World Health Organization (WHO) guide to analgesia includes nonsteroidal anti-inflammatory agents, narcotic analgesics, or adjuvant pain medicines, such as corticosteroids, nerve-stabilizing medicines, or antidepressants.23
RT palliates other local tumor symptoms with varying frequencies. Bleeding from lung tumors can be palliated in 80% to 90% of patients. Bleeding from sources such as the stomach, bladder, uterus, and rectum can also be palliated. More complex thoracic symptoms such as cough and dyspnea are palliated with radiation but with lower rates of success. Cough and dyspnea are palliated in 60% to 90% and 40% to 60% of patients, respectively.24–29 Symptoms associated with locally advanced gynecologic or bladder cancers are palliated in 60% to 94% of patients.30,31 More details about the efficacy of palliative radiation in various settings are addressed in the chapters that follow.
Side effects of RT
Since the goal of palliative radiation is to relieve suffering, most palliative radiation regimens are well tolerated and designed to have few acute or long-term side effects. Acute side effects are generally mild and managed with conservative measures. With the exception of fatigue, they relate to the organs within the boundaries of the radiation field. The primary systemic side effect of radiation is fatigue. Local side effects include skin irritation, nausea, diarrhea, esophagitis, and mucositis. The total dose of radiation and the fraction size correlate with treatment toxicity. Side effects occur acutely, subacutely, or in the long-term.
By definition, late effects occur several months to years after RT. They are generally rare and irreversible. There is a correlation between a higher fraction size and the risk of late effects, though established palliative regimens have very low rates of serious late effects. Patients with advanced cancer typically do not survive long enough to be at significant risk for the development of serious late complications of RT. Improvements in systemic therapies may ultimately increase the expected survival for patients with advanced cancer and thus place them at risk of late complications that can be associated with higher daily doses of radiation. Given the modest total doses employed and the relatively limited life spans of patients with metastatic cancer, this has not been a significant problem.
GUIDELINES AND QUALITY MEASURES
The overwhelming evidence from multiple randomized trials suggests equivalence between single and multifraction approaches with increased cost and inconvenience associated with multifraction RT. More than 101 different dose regimens have been employed worldwide for this single clinical circumstance.32 Both the American Society for Radiation Oncology (ASTRO) and the American College of Radiology (ACR) have guidelines for the treatment of uncomplicated bone metastasis with four approved, equivalent fractionation schemes (8 Gy/1 fraction, 20 Gy/4 fractions, 24 Gy/6 fractions, and 30 Gy/10 fractions).33–35 The National Quality Forum (NQF) has established the use of one of these four fractionation schemes as a quality metric.36 The American Board of Internal Medicine’s “Choosing Wisely” campaign, a program designed to help physicians become better financial stewards of health care use, designates single fraction RT as the treatment of choice for painful uncomplicated bone metastasis.37 Table 1.2 lists the percentage of single fraction RT utilized in various clinical settings. There is great variability between locations and between physicians in a given practice location.38–42
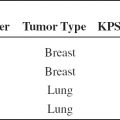
TABLE 1.2 Single Fraction RT Utilization
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree