The burden of chronic liver disease
Chronic liver disease (CLD) is a significant cause of morbidity and mortality and an important contributor to the burden of disease and health care utilization worldwide. It is estimated that CLD accounts for approximately 2 million deaths per year in the world: 1 million related to complications of cirrhosis and 1 million related to viral hepatitis and hepatocellular carcinoma (HCC); this is approximately 3.5% of all deaths worldwide. In a global estimation from 2019, CLD appeared in the top 10 causes of disability-adjusted life-years in men and ranked 16 in the general population. The current mortality attributed to liver disease as well as the prevalence of CLD and cirrhosis may be systematically underestimated in the United States.
The Global Burden of Disease study shows that age standardized incidence rate of CLD and cirrhosis has increased by 13% from 2000 to 2017. This increase is driven mostly by incidence of cirrhosis in Europe, high-income populations in Asia Pacific, East Asia, Southeast Asia, and South Asia. In the United States, a recent study suggests that, between 2007 and 2017, there has been a 30% increase in the incidence of cirrhosis and liver cancer. The driving force behind the reported increase in CLD is likely related to the raising rates of metabolic syndrome and alcohol misuse.
The epidemiology and distribution of the different causes of CLD varies geographically by ethnicity and by age group and is rapidly changing. From the 1.5 billion persons estimated to be affected with CLD worldwide in 2017, nonalcoholic fatty liver disease (NAFLD) accounted for 60%, hepatitis B 29%, hepatitis C 9%, and alcoholic liver disease 2%. Other far less common causes of CLD include autoimmune and cholestatic-related diseases such as autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, and metabolic/genetic diseases such as hemochromatosis.
NAFLD has a wide spectrum of disease including simple steatosis, nonalcoholic steatohepatitis (NASH), and cirrhosis. It is difficult to estimate the prevalence of the stages of NAFLD in the general population; based on radiological diagnosis (which cannot differentiate between simple steatosis, fibrosis, or cirrhosis), there is a worldwide estimated prevalence of 25% in NAFLD with the highest prevalence in the Middle East (32%), South America (31%), and Asia (34%). On a recent systematic review and metaanalysis of the prevalence of NAFLD in the United States, it is estimated that approximately one in three U.S. residents has steatosis, with higher rates seen in Hispanics; the prevalence of NASH among patients with NAFLD in the United States has been estimated to be around 32.4% based on histology and transaminases; and based on 11 studies assessing advanced fibrosis in patients with NAFLD, the prevalence was estimated to be close to 19%.
ALD also has a wide spectrum of disease, including alcoholic hepatitis, simple steatosis, steatohepatitis, and cirrhosis. The progression through these stages depends on continued alcohol use, comorbid liver disease, and other risk factors like female sex and genetic susceptibility. It is estimated that alcohol misuse accounts for 27% of deaths from liver disease and 30% of liver cancers in the world. , With an increase in alcohol consumption around the world, it is estimated that the burden of ALD will continue to increase.
Staging of liver fibrosis
CLD is characterized by progressive and diffuse deposition of fibrous tissue as a response of sustained liver injury or inflammation. This diffuse liver damage leads to architectural changes and replacement of the normal lobular structure of the liver parenchyma leading to the development of cirrhosis.
Histologically, fibrosis is a continuum, which has been graded in different stages; the most widely used staging system is the METAVIR fibrosis score, which is essentially the same as the NASH fibrosis score, and classifies the degree of fibrosis into five progressive stages: F0, no fibrosis; F1, portal fibrosis (or periportal fibrosis); F2, portal and periportal fibrosis with some septa formation; F3, bridging fibrosis; and F4, cirrhosis, characterized by the formation of nodules surrounded by fibrous tissue ( Fig. 1.1 ). ,
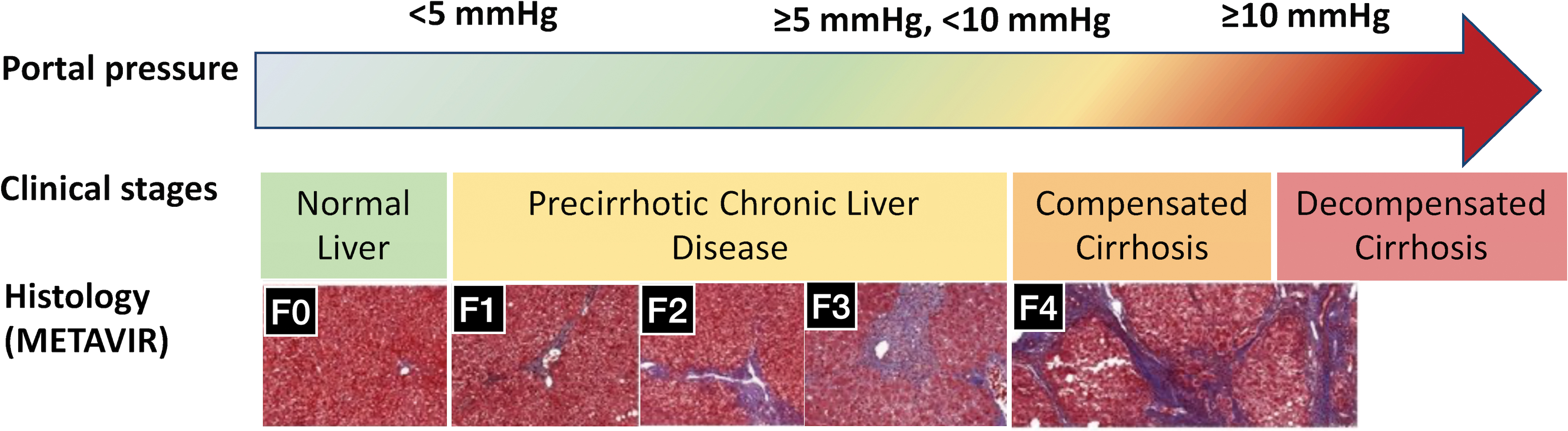
Stages of fibrosis, particularly stages 3 and 4 (cirrhotic stage), are important prognostically and are therefore important in identifying patients with CLD who would benefit from referral to a liver specialist, who would need to be prioritized for treatments of the underlying etiology of CLD, and in whom screening strategies would be initiated to prevent complications of CLD, specifically the development of complications of portal hypertension (ascites, variceal hemorrhage, encephalopathy) and the development of HCC.
Biopsy is the gold standard for staging of fibrosis. However, biopsies are an imperfect test. The accuracy of biopsy can be compromised by inadequate sampling, sampling variation, and interobserver variability at the time of interpretation. Sampling variation is the result of heterogenicity in inflammation and fibrosis across different etiologies of liver disease, and it has been shown to be a relevant issue in NAFLD, PBC, HCV, and HBV. Interobserver variability is not surprisingly an issue given that interpretation of liver histology is often complex; the degree of concordance varies according to the feature analyzed with degree of fibrosis and, although an adequate kappa ( k ) statistic of around 0.8 has been described for mostly viral etiologies of CLD, , , a more recent study showed important interobserver variability in the staging of fibrosis in patients with NASH. Furthermore, biopsies are an invasive procedure and, as such, they have been associated with complications such as pain, bleeding, bile duct injury, perforation of adjacent organs, infections, etc.
Stages of cirrhosis
Cirrhosis, which is the end stage of CLD of any etiology, is a histologic diagnosis based on the severity and burden of fibrosis, as described previously. Most complications of CLD, including liver-related complications (ascites, variceal hemorrhage, encephalopathy, or jaundice), HCC, and death, occur once the patient has reached a cirrhotic stage. Although patients with advanced fibrosis (F3) have also been described as having higher complication and mortality rates than those with earlier stages of fibrosis (F1, F2), the relevant fibrosis stage is the cirrhotic stage in which complications and liver-related mortality are highest. ,
Clinically, cirrhosis has been classified into two main stages: compensated and decompensated. , Decompensation in cirrhosis is defined by the development of overt ascites (not one identified only by ultrasound), variceal hemorrhage, and overt (not subclinical) hepatic encephalopathy and jaundice. Of these, those related to portal hypertension (ascites and variceal hemorrhage) are most common, whereas those related to liver insufficiency (encephalopathy and jaundice) occur more infrequently as, in the pathogenesis of cirrhosis, portal hypertension occurs first and liver insufficiency appears later on in the course of the disease. Once a patient develops one of these complications, the survival rate decreases significantly. In fact, the main determinant of death in patients with cirrhosis is the development of decompensation. The median survival in patients with compensated cirrhosis is typically greater than 12 years, as long as these subjects remain compensated, whereas the median survival rate in decompensated patients is under 2 years.
Compensated cirrhosis represents the long, asymptomatic stage of cirrhosis. Because these patients do not have overt complications of portal hypertension/liver insufficiency, the diagnosis is challenging. Several simple laboratory tests including platelet count, international normalized ratio (INR), albumin levels, and aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio, as well some other clinical findings such as splenomegaly, nodular liver, and presence of collaterals have varying predictive value in making a diagnosis of compensated cirrhosis. These parameters have been combined in different clinical scores like the Lok index, the FIB-4, or the NAFLD fibrosis scores to estimate the probabilities of cirrhosis. Although these variables/indices are valuable at ruling in cirrhosis, they are not optimal at ruling it out, resulting in the continued need to obtain a liver biopsy.
The diagnosis of cirrhosis in a decompensated patient is not as challenging; patients present with overt decompensating events, and, at this stage, they are more likely to have physical examination findings that support the presence of cirrhosis (e.g., palmar erythema, telangiectases, palpable left lobe of the liver, splenomegaly).
Another stage of cirrhosis that could be proposed is that of “regression” of cirrhosis. The process of fibrosis is dynamic, and cirrhosis is no longer considered an irreversible and progressive disease. There is evidence that, with treatment of the underlying liver disease (e.g., alcohol abstinence, antiviral therapy, immunosuppression in autoimmune diseases), fibrosis can regress, and in some cases of early cirrhosis it may regress to a precirrhotic stage. Patients with prior decompensated events can recompensate if the cause of disease is removed.
Clinically significant portal hypertension
Within the compensated stage, two substages have been recently identified based on the severity of portal hypertension.
The pathogenic substrate of decompensation in cirrhosis is related to portal hypertension. The structural change in the cirrhotic liver (fibrosis) together with active intrahepatic vasoconstriction lead to an increase in resistance to portal flow. This leads to mild portal hypertension, which, in turn, leads to splanchnic vasodilation with resultant increase portal venous inflow that further increases portal pressure.
Portal pressure can be measured indirectly by the hepatic venous pressure gradient (HVPG), which is a measure of hepatic sinusoidal pressure obtained by wedging (or balloon occluding) a catheter introduced via the jugular vein (or femoral vein) into one of the major hepatic veins (usually the right hepatic vein). In order to correct the wedged (occluded) pressure with a systemic pressure, a “free” pressure (with the catheter unwedged or with the balloon deflated) is subtracted from the wedged (occluded) pressure. Therefore the result is a gradient obtained by subtracting free from the wedged pressure. A normal HVPG is 3–5 mmHg. In most patients with cirrhosis, the HVPG is greater than 5 mmHg. When the HVPG reaches a threshold of ≥10 mmHg, patients with CLD have fourfold risk of decompensation compared to patients with cirrhosis and HVPG <10 mmHg. This HVPG ≥10 mmHg has also been associated to a greater probability of developing varices, HCC, postsurgical complications, and death. Therefore it is referred to as clinically significant portal hypertension (CSPH).
Patients with compensated cirrhosis are now subclassified into those with mild portal hypertension (HVPG >5 mmHg but <10 mmHg) and those with CSPH (HVPG >10 mmHg), with the latter being those more likely to decompensate and those in whom drugs that decrease portal venous inflow more effective.
In fact, the PREDESCI trial showed that, in patients with compensated cirrhosis and CSPH, decompensation can be prevented by using beta-blockers to decrease the portal pressure. Therefore the stratification of patients with advanced CLD, as those with CSPH and those without CSPH, has key implications for management of cirrhosis.
In the setting of compensated cirrhosis, elimination of the etiological agent delays decompensation of cirrhosis and progression of CLD, as mentioned, but it also has been proven to have a positive effect on HVPG, particularly in early stages of cirrhosis when patients have a lower degree of portal hypertension. However, it has been shown in subjects with cirrhosis related to HCV that, although there is an improvement in portal pressure, CSPH persists in the majority of patients, and therefore these patients appear to be at continued risk of decompensation and HCC. ,
As of now, the diagnosis of CSPH is established via hepatic vein catheterization. This is an invasive procedure that carries a risk, albeit small, of complications; furthermore, HVPG is a nuanced technique and appropriate, reliable measurements are not obtained in centers that do not perform the measurements routinely.
In conclusion, both the diagnosis of cirrhosis and that of CSPH require invasive procedures. The use of noninvasive means for staging, screening, and monitoring CLD, mainly the use of ultrasound-based technologies to determine liver stiffness, is currently favored and has become a cornerstone in the armamentarium of hepatologists around the world. Noninvasive means provide the advantages of safe longitudinal monitoring and the possibility of applying it to larger populations.
The objective of this book, therefore, will be to review the applications of parametric ultrasound in the context of the diagnosis, staging, and management of CLDs.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree