▪
Introduction
The focus of this chapter is to define terms used in cardiovascular and thoracic imaging and provide a basic framework for pattern recognition for problem solving in the clinical practice. The thoracic terms used in this book incorporate the recommendations of the Nomenclature Committee of the Fleischner Society published in 1984, 1996, and 2008 for thoracic radiography and computed tomography (CT). A few terms not included in the most recent Fleischner Society glossary of thoracic imaging terms are also defined. The updated terminology used in the cardiac and vascular imaging is also included.
Thoracic Terminology
Lung Parenchyma
Parenchyma: The gas-exchanging part of the lung is called the lung parenchyma and consists of the alveoli and their capillaries. On CT scans and radiographs, the lung parenchyma is the portion of the lung exclusive of visible pulmonary vessels and airways.
Lobe: Anatomically, lungs are divided into lobes, with the right lung typically consisting of three lobes and the left lung consisting of two lobes. Each lobe is enveloped by visceral pleura, except at the lung root (hilum) and in the presence of an incomplete interlobar fissure.
Segment: Anatomically, a segment refers to the lung lobe unit that is ventilated by a segmental bronchus, perfused by a segmental pulmonary artery, and drained by an intersegmental pulmonary vein. There are two to five segments per lung lobe.
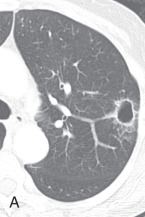
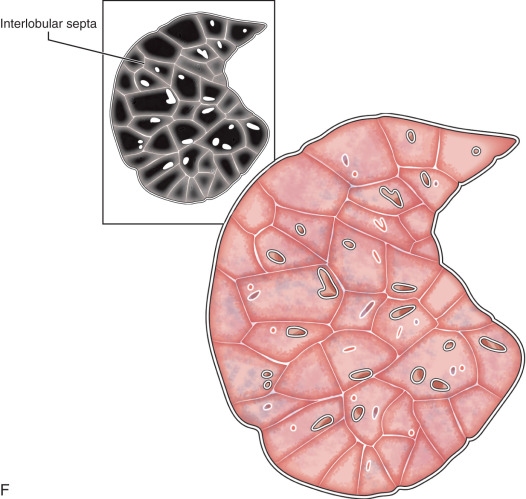
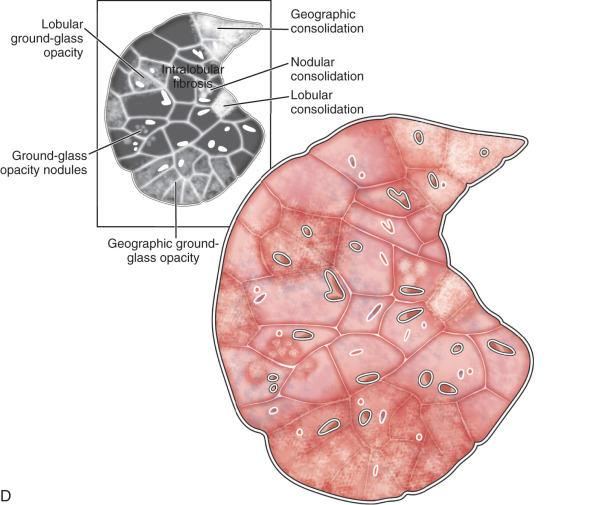
Lobule, secondary pulmonary lobule, pulmonary lobule: A secondary pulmonary lobule ( Fig. 13.1A ) is a fundamental unit of the lung, and the smallest, surrounded by connective tissue septa and interlobular septa. It is an irregular polyhedron in shape and varies from 1 to 2.5 cm in size. Each is more developed in the periphery of lung and in the anterior, lateral, and paramediastinal portions of the upper and middle lobes. Each secondary pulmonary lobule is supplied by small bronchioles and a pulmonary artery branch that enters in the center of the lobule; it is also known as a centrilobular or lobular core structure . The interlobular septae are composed of connective tissue and contain pulmonary venules and lymphatic channels. One secondary pulmonary lobule contains 3 to 25 acini.
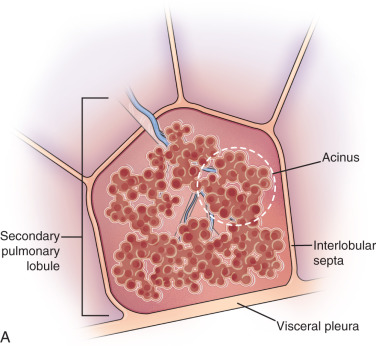
Figure 13.1
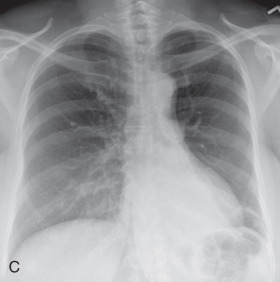
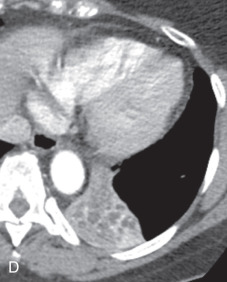
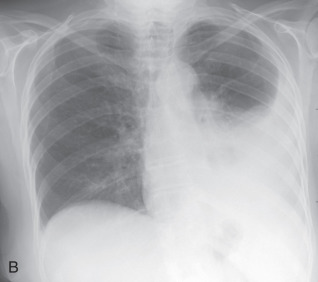
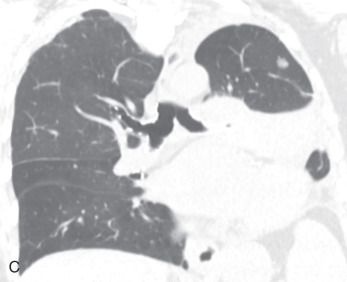
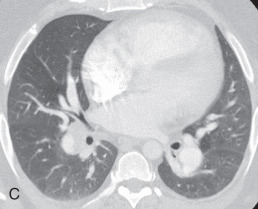
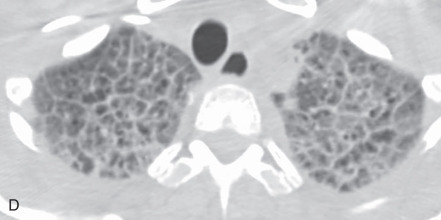
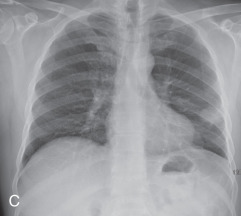
(A) Secondary pulmonary lobule. (B) A 62-year-old man with consolidation containing an air bronchogram in the posterior segments of both upper lobes and superior segments of both lower lobes, along with ground-glass opacities in the anterior segments of the right upper lobe and lingula due to multifocal pneumonia, which resulted in acute respiratory distress syndrome. Chest x-ray (C) and axial CT scan (D) in a 45-year-old man show a left lower lobe bronchial carcinoid causing its obstruction and retrocardiac opacification on a radiograph, correlating with a left lower lobe collapsed lung containing dilated, mucus-filled bronchi on the CT scan.
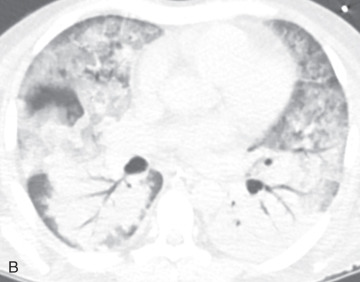
On thin section CT images, the secondary pulmonary lobule is composed of three primary components—the interlobular septa and septal structure, the centrilobular region and centrilobular structures, and the lobular parenchyma. In a normal lung, the pulmonary artery branch located in the center of secondary pulmonary lobule is visible (0.5–1.0 mm in diameter). However, a normally centrilobular bronchiole cannot be seen because of its thin wall (≈0.15 mm in diameter). The interlobular septa and septal structures are not usually visualized in the healthy lung (0.1 mm in the thickness) but are visible when thickened, such as in pulmonary edema and interstitial lung disease. Interlobular septa appear as thin linear opacities 10 to 20 mm in length between the secondary pulmonary lobules.
Lobular core structure: The lobular core structure at the center of secondary pulmonary lobule is composed of the centrilobular artery and preterminal bronchiole. The preterminal bronchiole branches into smaller preterminal bronchioles, terminal bronchioles, and respiratory bronchioles.
Acinus: There are 3 to 25 acini in one secondary pulmonary lobule. A acinus is the portion of the lung distal to a terminal bronchiole, which is the last purely conducting airway. The acinus is supplied by first-order respiratory bronchioles and contains alveolar ducts and alveoli. Because the respiratory bronchiole is the largest airway that contains alveoli in their walls, an acinus is the biggest pulmonary unit in which all airways participate in gas exchange. An acinus is usually 6 to 10 mm in diameter. On thin section CT, an acinus is not visible unless filled with pathologic material (e.g., exudates), which appear as poorly defined nodular opacities. Hence, on a CT scan, the acinar nodules appear as multiple 6- to 10-mm rounded and ill-defined pulmonary opacities. Acinar nodules and air space nodules are almost equivalent terms.
Air space: Air space refers to the air-containing lung, which includes respiratory bronchioles; however, it excludes purely conducting airways, such as terminal bronchioles.
Consolidation: Pathologically, consolidation refers to an exudative or other air space and interstitial disease process resulting in alveolar air replacement and an opacified lung. On radiographs and CT scans, consolidation refers to an increase in lung attenuation causing obscuration of the margins of underlying pulmonary vessels and airway walls (see Fig. 13.1B ). An air bronchogram may be present. The consolidation due to lipoid pneumonia shows decreased attenuation, and that related to pulmonary amiodarone toxicity has increased attenuation on CT and is helpful in narrowing the list of potential causes of the consolidation. Consolidation is nonspecific, with a wide differential diagnosis, so correlation with clinical and laboratory data is helpful.
Pneumonia: Pneumonia (see Fig. 13.1B ) is an inflammatory condition of the air spaces and interstitium. Infective pneumonia is an exudative pathology of the air space resulting in consolidation, such as bacterial pneumonia. Noninfectious pneumonia is the result of pulmonary inflammation and fibrosis, such as idiopathic interstitial pneumonia.
Bronchopneumonia, lobular pneumonia: Bronchopneumonia is characterized by infectious bronchitis, bronchiolitis, suppurative peribronchiolar inflammation, and subsequent patchy consolidation involving one or more secondary pulmonary lobules. It spreads through the airways and leads to thick and tenacious secretions in the bronchi and bronchioles. On the radiograph, bronchopneumonia appears as a multifocal, peribronchial, or patchy consolidation often in multilobar distribution. On CT, it appears as ill-defined centrilobular and tree-in-bud nodular opacities and lobular consolidation.
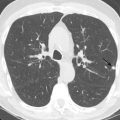
Atelectasis, collapse: Atelectasis is reduced inflation of all or part of the lung that leads to volume loss. A common mechanism is resorption of air distal to the obstructed airway due to mucus plugging or a mass. The term collapse is used synonymously with atelectasis, especially with its increased severity and increased lung opacification. On radiographs and CT scans, the direct signs of atelectasis (see Fig. 13.1C, D ) are a crowded pulmonary vasculature, crowded air bronchogram, and displacement of interlobar fissures. The indirect signs include pulmonary opacification, elevated diaphragm, ipsilateral shift of the heart and mediastinum, hilar displacement, compensatory hyperinflation of the adjacent lung, approximation of the ribs, and displaced pulmonary granulomas. Descriptively, atelectasis is classified into the following types: linear, subsegmental, segmental, lobar, or whole lung; rounded; and generalized or diffuse.
Linear atelectasis, discoid atelectasis, plate atelectasis: Linear atelectasis ( Fig. 13.2A ) is a focal subsegmental atelectasis with a linear shape that almost always abuts the pleura. It can be oriented in any plane from horizontal, oblique, to vertical, with a thickness ranging from a few millimeters to centimeters.
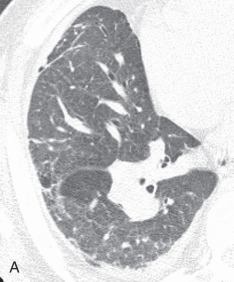
Figure 13.2
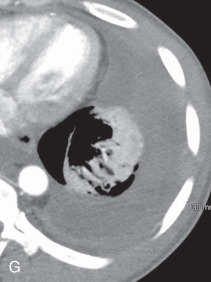
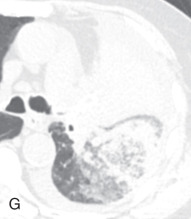
(A) Linear atelectasis, plate atelectasis, discoid atelectasis in a 61-year-old man with curvilinear parenchymal linear atelectasis in the right upper lobe. (B, C) Luftsichel sign. Chest x-ray (B) and coronal CT scan (C) in an 87-year-old man shows a left central bronchogenic carcinoma causing narrowing of the left upper lobe bronchus and resultant left upper lobe collapse, along with a hyperinflated left lower lobe in the form of the Luftsichel sign. Note the metastatic solid nodule in the hyperinflated left lower lobe. (D) Golden S sign in a 50-year-old man with collapse of the right upper lobe due to central obstructing squamous cell carcinoma of the lung, which gave rise to the Golden S sign. (E) Parenchymal band in a 61-year-old man with subpleural curvilinear parenchymal band related to prior asbestos exposure with adjacent pleural thickening. (F) Juxtaphrenic peak. (G) Rounded atelectasis in a 44-year-old man with enhancing rounded atelectasis in the left lower lobe along with empyema-related pleural thickening and pleural effusion in the left pleural cavity.
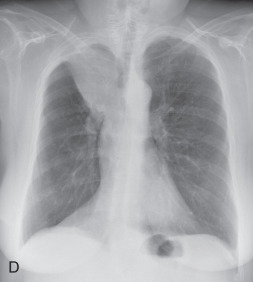
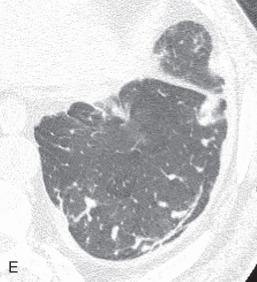
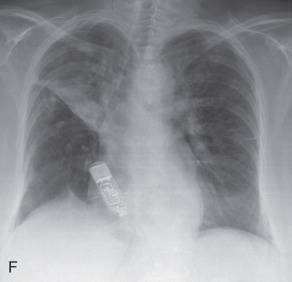
Luftsichel sign: The Luftsichel sign (from the German— luft means air, and sichel means sickle) is seen in left upper lung lobe collapse and occurs due to the upward and medial shift of the hyperexpanded superior segment of the left lower lobe, between the aortic arch and collapsed upper lobe. The collapsed left upper lobe moves anteriorly and superiorly to lie against the anterior chest wall; the hyperexpanded superior segment of the left lower lobe lies behind the upper lobe. On a radiograph (see Fig. 13.2B ), the Luftsichel sign is characterized as a sharply marginated, crescentic, paraaortic hyperlucency extending from the apex of the left hemithorax to the left superior pulmonary vein and outlining the medial aspect of the collapsed left upper lobe. On a CT scan (see Fig. 13.2C ), the collapsed left upper lobe is seen as a homogenous opacity lying anteriorly against the chest wall and mediastinum while the hyperexpanded superior segment of the lower lobe lies between the aortic arch medially and the collapsed upper lobe laterally, thus explaining the Luftsichel sign.
Golden S sign: The Golden S sign refers to right upper lobe collapse secondary to a centrally obstructing mass that is best seen on a frontal radiograph (see Fig. 13.2D ) and CT scans. The collapsed upper lobe shifts cephalad and medially while the central mass expands the hilum. The term inverted S sign of Golden is derived from the configuration of a minor fissure with an upward lateral convexity and a caudal medial convexity resembling the inverted S that is suggestive of a neoplastic cause. However, it is also seen in a right upper lobe bronchus obstruction due to enlarged central lymph nodes or a mediastinal mass.
Parenchymal bands: These represent linear pleuroparenchymal opacities due to fibrosis extending to the visceral pleural surface, which is often thickened and retracted at the site of contact (see Fig. 13.2E ). They are 1 to 3 mm thick and up to 5 cm long. They can represent contiguously thickened interlobular septa, peribronchovascular fibrosis, coarse scars, or atelectasis associated with lung or pleural fibrosis. They can be seen in patients with fibrosis and other causes of interstitial thickening such as asbestosis, silicosis, and sarcoidosis.
Juxtaphrenic peak: This is the tented or peaked appearance of the hemidiaphragm seen in the upper lobe or combined upper and middle lobe collapse or upper lobectomy. The sign is caused by retraction of the diaphragm at the inferior accessory fissure (most common), major fissure, or inferior pulmonary ligament.
On a frontal chest film, it appears as a small, sharply defined triangular opacity projecting upward from the highest point of the hemidiaphragm (see Fig. 13.2F ).
Rounded atelectasis, folded lung syndrome, Blesovsky syndrome, atelectatic pseudotumor: This is a unique type of chronic peripheral lung collapse that develops as a result of pleural fibrosis and that can mimic lung carcinoma. It is usually seen following healing of an asbestos exposure–related exudative pleural effusion, with pleural fibrosis and adjacent lung collapse, but can be seen in other conditions as well. It is usually detected as an incidental finding on imaging. On a radiograph, a rounded atelectasis appears as a peripheral round, oval, or wedge-shaped, peripheral, masslike opacity, commonly in the posterior part of the lower lobe, with curvilinear opacities from the bronchi and vessels (comet tail sign) extending from the site of the rounded atelectasis toward the hilum. Adjacent pleural thickening and volume loss of the affected lobe are always present. CT better demonstrates the full extent of disease than a radiograph. On CT, a rounded atelectasis appears as a peripheral soft tissue density-enhancing mass, with smooth margins, except at the entry site of bronchi and vessel, and that forms an acute angle with the adjacent thickened pleura. The distortion and displacement of the converging bronchovasculature represent one of the diagnostic and commonly seen features (see Fig. 13.2G ).
Infiltrate: This term was used on radiographs or CT scans to describe pulmonary opacification caused by air space or interstitial lung disease. Because the use of the term infiltrate is debatable, it is no longer recommended and has been widely replaced by other terminology. The term opacity with important qualifiers is preferred.
Parenchymal opacity: Parenchymal opacification means an increase in lung attenuation. It may or may not obscure underlying lung vasculature and airway walls. The more accurate terms are consolidation and ground-glass opacity . Consolidation is increased opacification of the lung, which obscures underlying vessels and lung architecture, except in the presence of an air bronchogram (see Fig. 13.2B ). A ground-glass opacity is a subtle increase in lung attenuation, with a preserved margin of the underlying structures (see Fig. 13.2B ).
Opacity: Opacity represents a focal increase in lung attenuation, thereby appearing more opaque than the surrounding area. It is a nonspecific term because it does not suggest the size and pathologic nature of the finding. It may represent any abnormality, including but not limited to a nodule, mass, consolidation, or ground-glass opacity. Measurement of lung attenuation on noncontrast-enhanced CT in the area of opacity can lead to the specific diagnosis. Attenuation higher than muscle is due to calcium deposition in a metastatic pulmonary calcification and pulmonary alveolar microlithiasis, talc powder deposits in talcosis, and iodine deposition of amiodarone toxicity. Attenuation less than muscle can be seen in lipoid pneumonia.
CT angiogram sign: The CT angiogram sign represents a finding in patients with consolidation on contrast-enhanced CT. It refers to the prominence of the contrast-opacified vessels in the consolidated lung ( Fig. 13.3A ). Although initially thought to be specific for bronchoalveolar carcinoma, it can be seen in pulmonary lymphoma and infectious and postobstructive pneumonia.
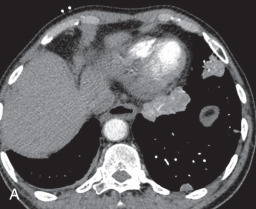
Figure 13.3
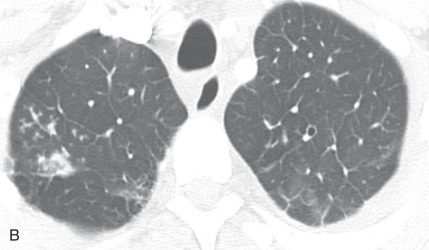
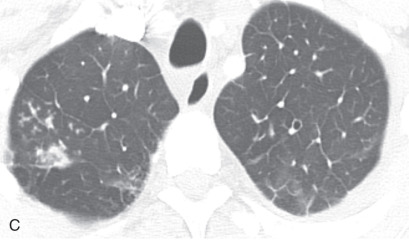
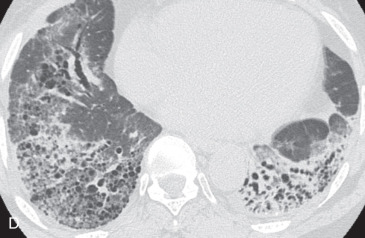
(A) A 50-year-old man with metastatic lung cancer causing multiple irregular enhancing nodular metastatic opacites in the left lung containing enhancing vessels suggestive of the CT angiogram sign. Note the right lower posterior pleural metastatic thickening. (B) In a 25-year-old man with cystic fibrosis, the CT scan reveals a mosaic attenuation pattern in the right lung due to cytomegalovirus-related small airway infection. Note the right middle lobe bronchiectasis, collapse consolidation of left lower lobe, and left pleural effusion. (C) A 50-year-old man with mosaic attenuation changes in both lungs due to pulmonary arterial hypertension. Note dilated pulmonary arteries. (D) A 50-year-old woman with an upper lobe, predominantly crazy paving pattern, and worsening hypoxia due to a combination of atypical pneumonia and pulmonary edema. The differential diagnosis include acute interstitial pneumonia, pulmonary edema, acute respiratory distress syndrome, and pulmonary hemorrhage. (E, F) Air trapping. Shown are extensive mosaic attenuation changes in both lungs on an inspiratory scan (E), with multiple patchy areas of air trapping on expiratory CT scans (F). The differential diagnoses include bronchiolitis obliterans, hypersensitivity pneumonitis, and chronic airway disease. (G) Organizing pneumonia in the left lower lobe in a 49-year-old man with distal left main bronchus carcinoma–related narrowing causing collapse of the left upper lobe and reversed halo sign (atoll sign) in part of the left lobe due to organizing pneumonia from a fungal infection.
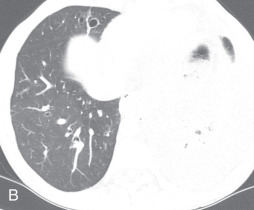
Ground-glass opacity: Ground-glass opacity (GGO) is a nonspecific term used to describe an increase in lung attenuation caused by a change in the relative proportions of air in the lung. This appearance is observed with air space disease causing partial filling of the alveoli with fluid, macrophages, neutrophils, or amorphous material, interstitial thickening due to inflammation, infiltration or fibrosis, partial alveolar collapse (atelectasis), and increased capillary blood volume. The degree of increase in lung opacity is not sufficient to obscure pulmonary vessels, as would be the case in pulmonary consolidation. Some active but potentially reversible processes that produce a GGO include pulmonary edema, pulmonary hemorrhage, alveolar proteinosis, and various causes of alveolitis and interstitial pneumonitis, whereas fibrosis is the leading cause of irreversible opacity. On a chest radiograph, a GGO appears as haziness of the lung, with indistinct bronchial or vascular markings. On a CT scan, it appears as a hazy increased lung opacity, without obscuring underlying bronchovascular margins (see Fig. 13.1B ).
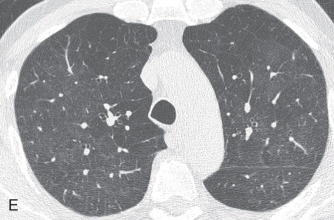
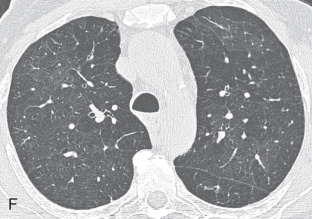
Mosaic perfusion, mosaic oligemia , mosaic attenuation pattern: The term mosaic attenuation pattern refers to an inhomogeneous lung opacity resulting in differing lung attenuation due to regional differences in lung perfusion on inspiratory CT. The causes are as follows: (1) occlusive vascular disease and vasculitis (see Fig. 13.3C ); (2) obstructive airway disease (see Fig. 13.3B ); or (3) mixed disease. Mosaic attenuation pattern is a more inclusive term than mosaic oligemia or mosaic perfusion. In mosaic perfusion, pulmonary vessels in low-attenuation lucent regions have a smaller caliber than in denser lung regions, thus differentiating them from a GGO. Redistribution of blood flow toward normal parts of the lung leads to increased attenuation of the uninvolved lung. Expiratory high-resolution computed tomography (HRCT) is useful in distinguishing mosaic perfusion due to airway disease from a vascular cause by demonstrating air trapping and sometimes thickened and dilated airways.
Head cheese sign: It represents the heterogeneous attenuation of the lung from a combination of mosaic perfusion and air trapping intermixed with the healthy lung. It is typically seen in hypersensitivity pneumonitis but has also been described in a few others, including sarcoidosis, atypical pneumonia, and respiratory bronchiolitis.
Crazy paving pattern: A crazy paving pattern refers to a GGO interspersed with a reticular pattern due to thickened interlobular septa or an intralobular interstitium (see Fig. 13.3D ). Initially described in alveolar proteinosis, it can also be seen in various acute and subacute conditions, including atypical pneumonia, pulmonary hemorrhage, diffuse alveolar damage, and organizing pneumonia.
Air trapping: Air trapping represents abnormal retention of air within the lung or part of the lung due to airway obstruction. On end-expiratory CT, the areas of air trapping are more evident and appear as darker or more radiolucent compared to normal lung, which has relatively high attenuation. Comparison between inspiratory (see Fig. 13.3E ) and expiratory CT (see Fig. 13.3F ) is required to detect air trapping.
Air bronchogram: On a radiograph and CT scan, an air bronchogram is a pattern of air-filled bronchus on a background of surrounding high-attenuation lung due to consolidation or ground-glass opacification. On the radiograph, an air bronchogram with opacity reliably separates an intrapulmonary location from a pleural or mediastinal abnormality. The sign is particularly well seen on CT scans (see Fig. 13.1B ) and indicates that proximal airways are patent but the alveolar space is filled (exudate, fluid, or cell), reduced (atelectasis), or a combination of both. The most common cause of an air bronchogram is the consolidation of various causative factors and pulmonary edema. It can also be seen in adenocarcinoma or marked interstitial lung expansion, as in lymphoma causing compression of the lung parenchyma, with patent airways.
Organizing pneumonia: Organizing pneumonia (OP) is characterized histologically by the presence of polyploid plugs of loose connective tissue and granulation tissue within the terminal or respiratory bronchioles, alveolar ducts, and alveoli, but with minimal or absent interstitial inflammation and fibrosis. OP is a relatively common condition accounting for approximately half of the cases of idiopathic interstitial pneumonia. Its idiopathic form is called cryptogenic organizing pneumonia . However, half of the histologic patterns of OP are secondary to conditions such as pulmonary infection, pulmonary infarction, hypersensitivity pneumonitis, collagen vascular disease, and a drug reaction.
On the radiograph, OP appears as patchy peripheral and basal consolidative opacities. On CT scans, the typical pattern is unilateral or bilateral, patchy, nonsegmental, often triangular or polygonal air space consolidation in a subpleural and peribronchovascular distribution and often in the lower lobes (see Fig. 13.3G ). An air bronchogram may be present. Scattered GGOs, centrilobular nodules, or a tree-in-bud pattern can also be seen. The lesions tend to progress and migrate over time. The differential diagnoses include bronchioalveolar carcinoma, lymphoma, vasculitis, and infections.
Diffuse alveolar damage: See acute interstitial pneumonia.
Pulmonary laceration: A pulmonary laceration is characterized by disruption of the lung parenchyma, resulting in a round or oval pulmonary cavity. The traumatic cavity may be filled with air (traumatic pneumatocele), blood (traumatic hematocele), or both (hematopneumatocele). Adjacent lung contusions are usually present, which obscure its identification on the radiograph. It is usually seen in children and young adults due to more flexibility of the chest wall. According to the mechanism, four CT patterns of lung injury ( Fig. 13.4A ) can be seen:
Type 1, compression rupture injury: Direct compression force results in laceration in a deep portion of the lung.
Type 2, compression shear injury: A severe sudden blow to the lower hemithorax results in the sudden shift of the lower lobes of the lung across the spine. These are usually paraspinal in location.
Type 3, rib penetration tear: This is peripheral in location, near a displaced rib fracture, and is associated with pneumothorax.
Type 4, adhesion tear: This is a laceration in the region of preexisting pleuroparenchymal adhesions and is usually diagnosed at surgery or autopsy.
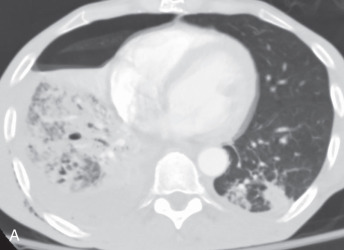
Figure 13.4
(A) Pulmonary laceration—a 56-year-old man with a motor vehicle collision–related right lower lobe contusion and laceration, patchy left lower lobe contusion related opacities, and a moderate-sized right hydropneumothorax. (B) Interlobular septal thickening, beaded septum sign, septal thickening in a 23-year-old man with cystic fibrosis. Note the ill-defined centrilobular opacities in the apices of both lungs with tree-in-bud opacities in the right apex proven to be from Aspergillus infection, along with mild bronchiectasis (not shown). The mild smooth interlobular septal thickening in both lung apices is due to associated pulmonary edema. (C) Subpleural curvilinear line—a 61-year-old woman with usual interstitial pneumonia (UIP)–related mild subpleural right upper lobe lines and bilateral subpleural reticular opacities. (D) Architectural distortion—a 66-year-old woman with UIP presenting with honeycombing reticular opacities, architectural distortion, traction bronchiectasis, bronchiolectasis, and mild ground-glass opacities predominantly in both lower lobes. (E) Reticular pattern—a 40-year-old woman with lymphocytic interstitial pneumonia–related, bilateral lower zone, predominantly reticular pattern opacities caused by multiple lung cysts and reticulation. (F) Schematic illustration demonstrating perilobular pattern causing perilobular thickening. (G) Reticulonodular pattern—a 70-year-old woman with sarcoidosis; right upper lobe opacity is related to collapse consolidation due to chronic partial right and middle upper lobe, peribronchial, soft tissue causing narrowing, with resultant elevation of the right dome of the diaphragm and juxtaphrenic peak. Note the bilateral reticulonodular opacities in both lungs from sarcoidosis.
Pulmonary contusion: Pulmonary contusion is the most common lung injury caused by blunt chest trauma. It is a traumatic alveolar injury with an alveolar hemorrhage, but without significant alveolar disruption. It usually occurs within 24 hours of injury, usually at the site of impact. On the radiograph, the typical imaging feature is a patchy, ill-defined consolidative opacity in the nonsegmental pattern. On CT scans, subpleural sparing (1–2 mm of clear parenchyma beneath the pleural surface) may be observed ( Fig. 13.4A ). CT is more sensitive for the detection of contusion because the contusion is seen immediately after injury on a CT scan but may not be seen until 6 hours after injury on a radiograph.
Interstitium
Interstitium: The pulmonary interstitium is the continuum of connective tissue throughout the lung and comprises three subdivisions:
- 1.
The bronchovascular (axial) interstitium, surrounding and supporting the bronchi, arteries, and veins from the hilum to the level of the respiratory bronchiole
- 2.
The parenchymal (acinar) interstitium, situated between the alveolar and capillary basement membrane
- 3.
The subpleural connective tissue contiguous with the interlobular septa (see Fig. 13.4A )
- 1.
Interlobular septum: See secondary pulmonary lobule.
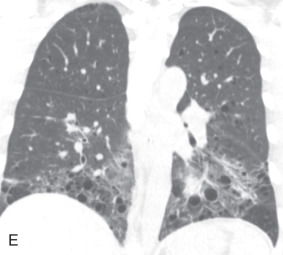
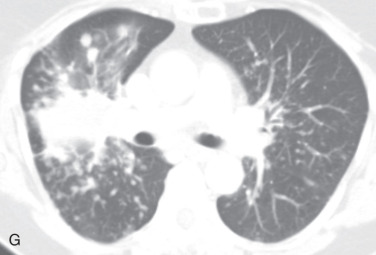
Interlobular septal thickening, beaded septum sign, septal thickening: Thickening of the interlobular septum is easily recognized on HRCT. On a chest radiograph, they appear as a thin linear opacity at a right angle to and in contact with the lateral pleural surfaces near the lung bases (Kerley B lines). Kerley A lines seen in the upper lobes are 2- to 6-cm long thin lines, with the radial orientation toward the hilum. Recently, the anatomic descriptive term interlobular septal lines or septal thickening has gained preference over Kerley lines. On HRCT, the normal interlobular septum is not visible but, in several pathologic conditions, septal thickening is seen. Four different types of septal thickening are noted, based on the appearance and underlying cause. Pulmonary edema results in smooth septal thickening (see Figs. 13.3D and 13.4B ). Infiltrative diseases, such as a tumor or tumor-like process, cause nodular septal thickening—a beaded septum sign of the lymphangitic spread of malignancy and sarcoidosis. Fibrotic conditions, such as interstitial fibrosis, cause irregular septal thickening (see Fig. 13.4C ) and marked lung architecture distortion.
Intralobular lines, intralobular interstitial thickening: Intralobular lines are fine linear opacities within the secondary pulmonary lobule, which, when thickened, appear reticular or meshlike on HRCT (see Fig. 13.4C ). These are seen in various disease processes, such as interstitial pulmonary fibrosis, alveolar proteinosis, and pulmonary hemorrhage.
Subpleural curvilinear line: This is seen on CT as a thin, nonspecific, curvilinear opacity, 1 to 3 mm in thickness, lying parallel to and less than 1 cm from the pleura (see Fig. 13.4C ). It can be a normal finding when seen in the dependent parts of the lung and represents dependent atelectasis; it disappears on a prone CT scan. Curvilinear lines are also observed in fibrosis, pulmonary edema, and asbestosis.
Architectural distortion: This term refers to a distorted appearance of the lung due to the abnormal displacement of pulmonary structures, such as bronchi, vessels, fissures, or interlobular septa. It can be caused by diffuse or localized lung disease, particularly lung fibrosis (see Fig. 13.4D ) or volume loss of the lung.
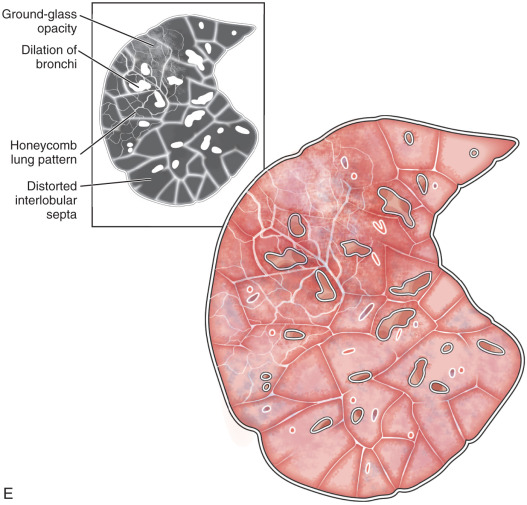
Honeycombing: Honeycombing occurs due to alveolar disruption and dilation of alveolar ducts, with the loss of acinar architecture. It represents destroyed and fibrotic lung tissue. Honeycombing on HRCT is characterized by the presence of peripheral, subpleural, air-filled cysts, typically between 3 and 10 mm in diameter. However, it may be as large as 2.5 cm, with clearly defined fibrous walls of 1 to 3 mm and arranged as a few clustered cysts in the early stage to multilayered cysts in the advanced stage (see Fig. 13.4D ). It is a feature of established pulmonary fibrosis and is an important criterion for the diagnosis of usual interstitial pneumonia.
Reticular pattern, reticulation: On chest radiographs, a reticular pattern consists of innumerable linear opacities that resemble a mesh or net due to a summation effect (see Fig. 13.4E ). A reticular pattern usually represents interstitial lung disease. It is a purely descriptive term, and several morphologic variations are seen, ranging from generalized interlobular septal thickening to intralobular lines to honeycomb lung destruction or parenchymal bands or irregular linear opacities. Reticular interstitial septal thickening can be smooth (pulmonary edema; see Fig. 13.4B ), nodular (lymphangitic spread of malignancy, sarcoidosis), or irregular (fibrosis; see Fig. 13.4D ).
Perilobular pattern: The perilobular region is composed of the structures that border the periphery of the secondary pulmonary lobule, which include the interlobular septa, visceral pleura, and vessels. Hence, the pulmonary disease occurring predominantly with the interlobular septa and periphery of the secondary pulmonary lobules has the perilobular pattern. On CT, the perilobular pattern is an ill-defined arcuate or polygonal bandlike opacity of thickness greater than that of thickened interlobular septa. These may be associated with ground-glass or consolidative opacities. The term is frequently used in the context of perilobular distribution of OP.
Centrilobular pattern: The centrilobular region represents the bronchiolovascular core of a secondary pulmonary lobule and consists of lesions that have an effect beyond the terminal bronchioles that center on the respiratory bronchioles or even the alveolar ducts. On CT, the centrilobular region appears as a small dotlike or linear opacity due to the intralobular artery in the center of a secondary pulmonary lobule, most evident within 1 cm of a pleural surface. Centrilobular disease patterns consist of (1) nodules, (2) tree-in-bud opacities due to small-airway disease (see Fig. 13.4B ), or (3) an abnormal low attenuation pattern due to centrilobular emphysema.
Reticulonodular pattern: The reticulonodular pattern represents the combination of the reticular and nodular patterns on the radiograph and is usually the result of the intersection of innumerable lines, creating the effect of additional micronodules (see Fig. 13.2F and G ). The dimension of the nodule is related to the size and number of linear or curvilinear components. (See also the reticular pattern.) The reticulonodular pattern appears on CT scans due to the simultaneous presence of reticular and micronodular patterns. On CT, the micronodules can be located in the center of the reticular elements, suggesting centrilobular micronodules, or superimposed on the linear opacities, suggesting septal micronodules.
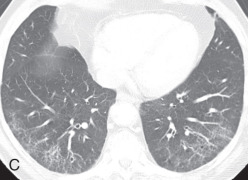
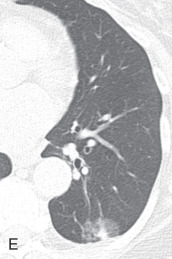
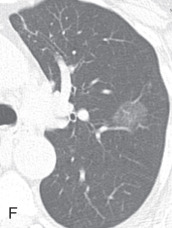
Respiratory bronchiolitis, respiratory bronchiolitis – interstitial lung disease: Respiratory bronchiolitis (RB) and respiratory bronchiolitis–interstitial lung disease (RB-ILD) are part of a spectrum of smoking-related inflammatory diseases of the small airways and lungs. In the order of increasing severity, first is RB, then RB-ILD, and then desquamative interstitial pneumonia. Symptomatic smokers with RB-ILD histologically show an exaggerated form of RB, with more extensive inflammation and fibrosis. Chest radiographs are mostly normal in patients with RB or RB-ILD. HRCT findings in RB ( Fig. 13.5A ) and RB-ILD include poorly defined centrilobular nodules and patchy, multifocal, ground-glass opacities with mid and upper lung predominance, with or without accompanying areas of patchy centrilobular emphysema. Also, RB-ILD patients can have bronchial wall thickening and fibrosis-related reticular opacities.
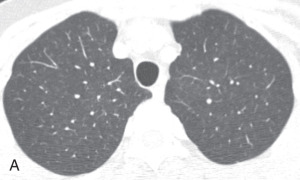
Figure 13.5
(A) Respiratory bronchiolitis (RB) and RB interstitial lung disease—a 49-year-old woman with RB-related bilateral upper lobe, ill-defined centrilobular ground-glass opacities (GGOs). She had a smoking history of 30 years. (B) Acute interstitial pneumonia (AIP)—a 49-year-old woman with AIP-related diffuse mixed ground-glass and consolidative opacification in both lungs and barotrauma-related loculated right pneumothorax and small pneumomediastinum. (C) Desquamative interstitial pneumonia (DIP)—a 70-year-old man with chronic smoking with DIP changes in the form of bilateral fine reticular and ill-defined GGOs with subpleural sparing. (D) Lymphoid interstitial pneumonia—a 40-year-old woman with dyspnea shows multiple focal round variable-sized, air-filled lucencies with thin walls in the lung parenchyma, with an apical basilar gradient with predominantly lower lobe involvement. Note the associated peribronchovascular consolidations in the lower lobes and inferior right middle lobe. These findings were proven to be due to lymphocytic interstitial pneumonitis, whereas the consolidations can occur because of superimposed infection, lymphoma, or organizing pneumonia. (E) Schematic illustration demonstrating various manifestations of usual interstitial pneumonia (UIP). However, ground glass-opacities are extremely rare in UIP. (F) Nonspecific interstitial pneumonia (NSIP)—a 48-year-old woman with hypoxemic respiratory failure and history of polymyositis. CT scan shows bilateral predominantly peribronchovascular and subpleural GGOs, interlobular and intralobular septal thickening with upper lobe predominance, and minimal traction bronchiectasis; no honeycombing seen. These findings were pathologically shown to be from a mixed type of NSIP.
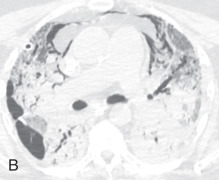
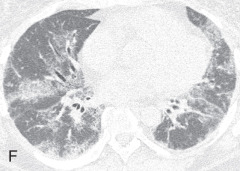
Acute interstitial pneumonia: Acute interstitial pneumonia (AIP) is a fulminant disease of unknown cause, usually occurring in a previously healthy person. This is associated with the histologic pattern of diffuse alveolar damage. Chest radiographs show diffuse or lower lung zones with predominantly bilateral air space consolidation, with an air bronchogram or GGO. Honeycombing may be seen late in the disease. Findings are similar to other causes of acute respiratory distress syndrome (ARDS). On CT, the early exudative phase of AIP is characterized by bilateral, patchy, GGOs and consolidation, predominantly of the dependent lung and sometimes with a crazy paving pattern (see Fig. 13.5B ). The later organizing phase of AIP is associated with distortion of bronchovascular bundles and traction bronchiectasis due to evolution to fibrosis. Areas of honeycombing are seen in the chronic phase occurring after 2 weeks in survivors.
Desquamative interstitial pneumonia: Desquamative interstitial pneumonia (DIP) is a rare interstitial lung disease primarily affecting smokers but can also be seen in various other conditions, such as occupational dust exposure, drug reaction, and infection. The predominant pathologic feature is the intraalveolar and distal airway accumulation of pigmented macrophages, with mild inflammation and minimal fibrosis. The macrophages are uniformly and diffusely distributed, unlike the bronchocentric distribution in RB-ILD. The chest radiographic appearances of DIP are nonspecific and reveal predominant GGOs in the lower lung zone. On HRCT, DIP is characterized by bilateral, patchy, GGOs, with subpleural and basal predominance (see Fig. 13.5C ), air-filled cysts, centrilobular emphysema, mosaic perfusion, and mild reticular opacities. However, minimal honeycombing may also be present. It can be indistinguishable from RB-ILD.
Lymphoid interstitial pneumonia: Lymphoid interstitial pneumonia (LIP) is a rare interstitial pneumonia and is usually a secondary disease due to conditions such as Sjögren syndrome, HIV infection, and various immunodeficiency syndromes. It is characterized by diffuse infiltration of the interstitium by lymphocytes, plasma cells, and histiocytes and by diffuse peribronchiolar inflammation. The chest radiograph shows nonspecific bilateral reticular, reticulonodular, and air space opacities. On HRCT, diffuse, bilateral, GGOs, ill-defined centrilobular opacities, subpleural and peribronchovascular nodules, and thin-walled cystic spaces are the predominant findings (see Fig. 13.5D ).
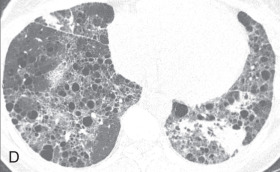
Usual interstitial pneumonia: Usual interstitial pneumonia (UIP) is the most common interstitial pneumonia and can be idiopathic or secondary to a drug reaction, asbestosis, or collagen vascular disease. UIP is characterized by temporal and spatial heterogeneity, with areas of normal lung alternating with interstitial inflammation and honeycombing (see Fig. 13.5E ). The key findings are fibroblastic foci and fibroblastic destruction of the lung architecture, with macrocystic honeycombing. It has subpleural and basal lung zone predominance.
In the early stage, the radiograph is normal. In the advanced stage, the radiograph shows decreased lung volumes and predominant subpleural irregular reticular opacities in the lower zone and honeycombing. On HRCT, patchy, peripheral, and subpleural reticular opacities, traction bronchiectasis, macrocystic honeycombing with an apicobasal gradient, and areas of mild GGOs are highly suggestive of UIP (see Fig. 13.4D ).
Idiopathic pulmonary fibrosis: Idiopathic pulmonary fibrosis (IPF) is a specific form of chronic, progressive, fibrosing interstitial pneumonia of unknown cause, limited to the lungs and associated with a histopathologic and/or radiologic pattern of UIP. On HRCT, IPF is characterized by the presence of bilateral, basal, peripheral subpleural reticular opacities associated with traction bronchiectasis (see Fig. 13.4D ). Honeycombing is common and is critical for making a definitive diagnosis of IPF. Temporal and spatial heterogeneity due to old and active fibrotic processes and the presence of fibroblastic foci are pathologic characteristics of IPF.
Nonspecific interstitial pneumonia: Nonspecific interstitial pneumonia (NSIP) is a chronic interstitial lung disease characterized by interstitial inflammation and fibrosis. It can be idiopathic or due to connective tissue disorders, hypersensitivity pneumonitis, or a drug reaction. The characteristic histologic pattern of temporal and spatial homogeneous lung involvement distinguishes it from UIP.
On the radiograph, GGOs, predominantly in the lower lobes, along with reticular opacities, may be seen. HRCT findings include involvement of the lower lung lobes, but the sharp apicobasal gradient is absent. Bilateral symmetric patchy or subpleural GGOs with irregular linear or reticular opacities, with a relative subpleural dorsal lung sparing, and traction bronchiectasis are common findings. In advanced disease, traction bronchiectasis and patchy consolidation can be seen (see Fig. 13.5F ).
Nodule
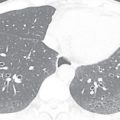
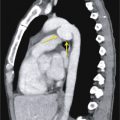
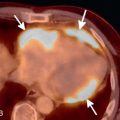
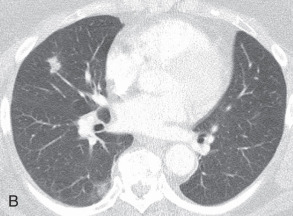
Solitary pulmonary nodule: A solitary pulmonary nodule (SPN) is a relatively well-defined, roughly spherical focal opacity, which is at least partially surrounded by the lungs, and is 3 cm or less in diameter ( Fig. 13.6A–C ). The most important first step is to determine that the nodule is truly solitary and arises from the lung. The differential diagnoses of SPN are extensive, and most fall under malignant and benign neoplasms and infectious granulomas. In a patient with SPN, a plain film or CT scan is used to determine the following: (1) morphologic features (e.g., margin, size, shape); (2) density or attenuation (e.g., solid, part solid, ground glass; fat, calcium, or contrast enhancement); and (3) growth rate.
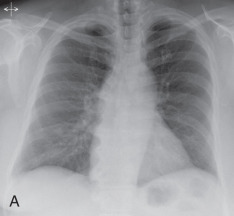
Figure 13.6
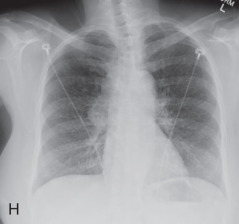
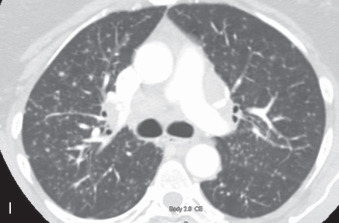
(A) Solitary pulmonary nodule. (B) A 61-year-old woman with stage I cervical cancer shows a faintly visualized nodular opacity in the right midzone overlapping the right anterior fourth rib, which correlates with the right middle lobe nodular opacity on the CT. Biopsy of this lesion revealed primary squamous cell carcinoma of the lung. (C) A 61-year-old man with a solitary nodular mass in the left midzone, which was confirmed to be a large 3.5-cm pulmonary arteriovenous malformation on the CT. (D) Schematic diagram showing various types of lung nodules and opacities. (E) A 63-year-old man with a 2.5-cm part solid nodule in the left lower lobe with a predominantly ground-glass component and a 0.7-cm solid component, which was biopsy-proven to be adenocarcinoma. (F) A 77-year-old man with a 2.3-cm ground-glass nodule in the left upper lobe, which was biopsy-proven to be adenocarcinoma. (G) A 63-year-old woman with a typical carcinoid presenting as a solid endobronchial nodule in the left lower lobe bronchus causing the left lower lobe to collapse; it contained mucus-filled dilated brochi. (H, I) A 58-year-old woman with diffuse miliary nodules in both lungs with centrilobular and subpleural distribution and enlarged mediastinal and bilateral hilar nodes, pathologically proven to be sarcoidosis. The differential diagnosis included tuberculosis, fungal infection, metastasis, and pneumoconiosis.
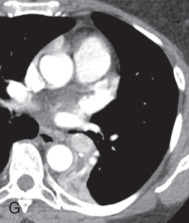
Nodule: On a chest radiograph, a nodule appears as a rounded opacity, with a well- or ill-defined margin, 3 cm or less in diameter. A pseudonodule includes a rib lesion, skin lesion, or external device, anatomic variants, or a summation lesion that may mimic a pulmonary nodule on a radiograph. On a CT scan, a nodule is a rounded or irregular opacity, with well- or ill-defined margins and less than 3 cm in diameter. A nodule can be solid (homogenous soft tissue attenuation), nonsolid (ground-glass attenuation), or part solid (consisting of both soft tissue and ground-glass attenuation; see Fig. 13.6D ). A nonsolid or ground-glass nodule (GGN) or GGO (see Fig. 13.6F ) is defined as a well-defined or poorly defined localized nodular area of increased lung attenuation through which normal parenchymal structures, including airways and vessels, can be visualized. A part solid GGN is defined as a lesion containing a combination of both ground-glass and solid components, with the latter component obscuring the underlying lung architecture (see Fig. 13.6E ). Purely solid nodules are defined as homogenous soft tissue attenuation lesions (see Fig. 13.6G ).
Micronodule: A micronodule is a discrete, small, round, focal opacity with a diameter of less than 5 mm, often less than 3 mm (see Fig. 13.6H, I ). It has been recommended by the Fleischner Society that this term be reserved for describing nodules that are less than 3 mm in diameter.
CT halo sign: The CT halo sign refers to a halo of GGO surrounding a solid pulmonary nodule, mass, or consolidation. In leukemic patients with angioinvasive aspergillosis, the halo sign indicates hemorrhage surrounding a focal septic infarct. In patients with tumor, particularly adenocarcinoma or bronchoalveolar carcinoma, it reflects the presence of lepidic spread of the tumor. The CT halo sign is nonspecific and may also be seen in various other pulmonary infections, infarction, and vasculitis.
CT reverse halo sign, atoll sign: The CT reverse halo sign is a focal area of GGO surrounded by a crescentic or complete ring of denser opacity (at least 2 mm in thickness; see Fig. 13.3G ). It was initially thought to be specific for cryptogenic OP but can be seen in other conditions, such as chronic eosinophilic pneumonia, various fungal and bacterial infections, infarct, and polyangiitis granulomatosis.
Feeding vessel sign: The feeding vessel sign refers to a small pulmonary artery leading directly to a pulmonary nodule. It is usually seen with hematogenous metastasis (see Fig. 13.3G ), but can also be seen in septic emboli, infarcts, and an arteriovenous fistula. It is rare with primary lung tumors or benign lesions such as granulomas.
Miliary pattern: The miliary pattern refers to numerous tiny (<3 mm in diameter), discrete, rounded pulmonary nodules that are usually uniform in size, diffuse, and randomly distributed throughout the lungs (see Fig. 13.6H, I ). This pattern of micronodules is characteristic of the hematogenous spread of tuberculosis and endemic fungi, metastatic disease, and pneumoconiosis. This pattern is seen on both CT and HRCT ( Fig. 13.7A ).