Fig. 6.1
Cardiac catheterization lab equipment. X-rays are emitted from the source and travel through the patient to the image intensifier (Reproduced with permission of Elsevier from Libby et al. [23])
Coronary angiography involves cannulation of coronary vessels using small, flexible catheters (typically 4 thru 6 French catheter diameters for diagnostic coronary angiography) and injection of radio-opaque iodinated contrast, allowing visualization of the coronary anatomy with x-ray fluoroscopy.
A variety of techniques and access sites may be used to gain entry to the arterial system for the performance of ICA. Today, most ICA are performed using a modification of the percutaneous femoral arterial approach originally suggested by Seldinger in 1953 (Fig. 6.2a, b).
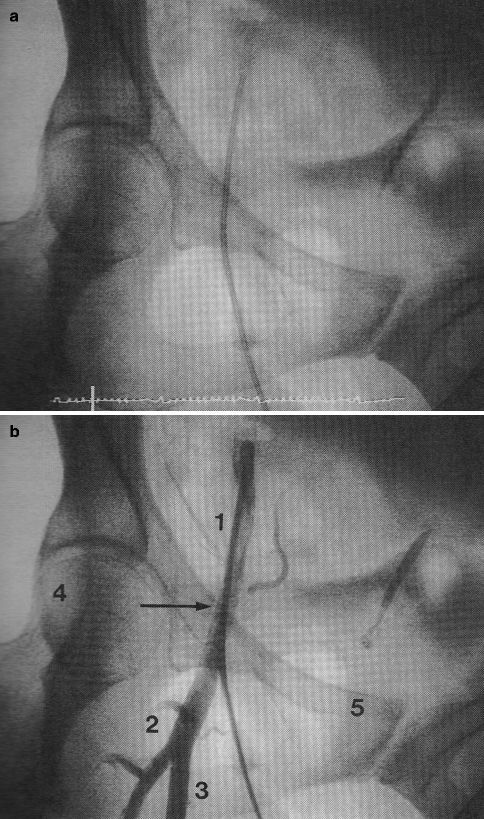

Fig. 6.2
(a, b) Femoral artery landmarks. (a) Angiogram sheath in femoral artery in anteroposterior projection. (b) Correct positioning is seen relative to angiographic landmarks: 1 common femoral artery, 2 bifurcation of profunda, 3 superficial femoral artery, 4 Midpoint of femoral head, 5 Iliac-symphysis pubis ridge (inguinal ligament line) (Reproduced with permission of Elsevier from Kern [24])
While the common femoral artery is the most common site for arterial access, however, the radial artery is increasingly being utilized as a site through which to perform invasive coronary angiography due to its inherent improved patient comfort due to ease of post-procedural hemostasis and studies demonstrating very high rates of operator success. Less commonly, a brachial arterial approach is employed.
After gaining arterial access and placement of an access sheath, a coronary catheter is advanced over a wire in a retrograde fashion into the ascending aorta and once the wire is removed, the coronary arteries are selectively engaged using fluoroscopic guidance. The left and right coronary artery systems are then selectively engaged and injected with iodinated contrast for anatomic visualization.
Anatomic visualization with invasive coronary angiography offers the advantage beyond all other coronary imaging modalities of possible conversion to a therapeutic percutaneous coronary intervention (PCI), if clinical indicated. However, as an invasive procedure, coronary angiography also conveys a higher risk (relative to other coronary imaging modalities) of death, myocardial ischemia and infarction, and arterial injury such as dissection, aneurysm, or arteriovenous fistula.
In addition, coronary angiography requires exposure to ionizing radiation (mean effective dose 6 mSv for diagnostic invasive coronary angiography) [6] and can result in iodinated contrast anaphylaxis or contrast induced nephropathy [7].
In spite of these risks, which are generally low on a per-patient basis, invasive coronary angiography has remained as the reference standard for coronary artery disease diagnosis and severity quantification since the 1960s Table 6.1.
Table 6.1
Catheter angiography vs. noninvasive imaging
Advantages | Disadvantages |
|---|---|
Percutaneous intervention possible | |
Possible use of fractional flow reserve (FFR) and intravascular ultrasound (IVUS) | May be more costly |
Monitored environment | Contrast media reactions |
Diagnostic “gold standard” | Risks of sedation |
No ischemic correlation without FFR |
Multiple views (Fig. 6.3a, b) of the coronary anatomy are typically taken due to commonly-encountered overlapping vessels and vessel foreshortening (erroneous perception of a shortened arterial segment due to camera angle) and in order to clearly distinguish artifact from true coronary artery lesions.
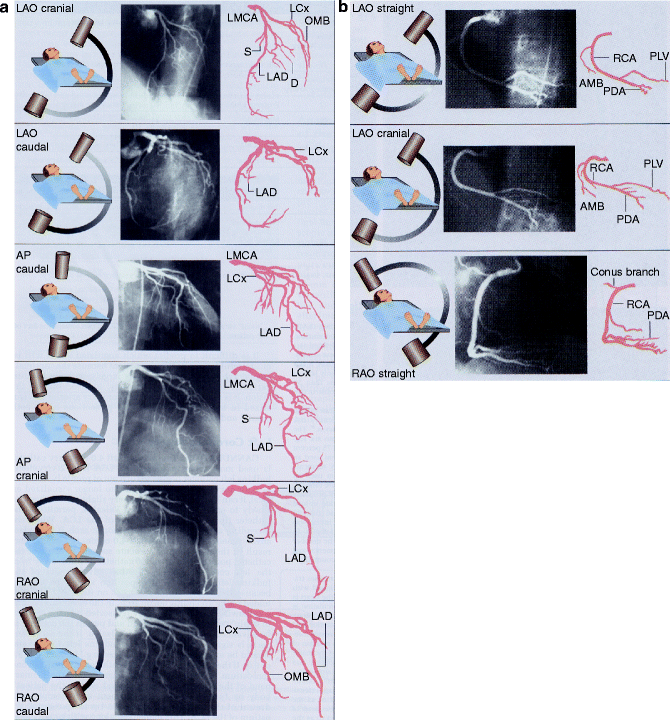

Fig. 6.3
(a, b) Standard views of the left and right coronary artery system. LAO left anterior oblique, AP anterior-posterior, RAO right anterior oblique (Reproduced with permission of Elsevier from Libby et al. [23])
A typical progression of camera views includes:
Left Coronary Artery
1.
Right anterior oblique (RAO)-caudal to visualize the left main, proximal LAD, and proximal circumflex
2.
RAO-cranial to visualize the mid and distal LAD without overlap of septal or diagonal branches
3.
LAO-cranial to visualize the mid and distal LAD in an orthogonal projection
4.
LAO-caudal to visualize the left main and proximal circumflex
Right Coronary Artery
1.
LAO to visualize the proximal right coronary artery (RCA)
2.
RAO-cranial to visualize the posterior descending and posterolateral branches
Quantification and Accuracy
Visual Estimation
Angiographer visual estimation of luminal stenosis is the most widely used method for quantification of coronary artery disease distribution and stenosis severity during ICA (Fig. 6.4).
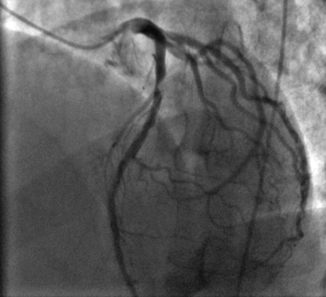
Fig. 6.4
Obstructive lesion in the proximal left anterior descending (LAD) artery in a patient who presented with anterior wall ST elevation myocardial infarction
The reliability of percent stenosis estimation compared with post-mortem studies is high: 79–93 % agreement is reported in studies that compared arterial segments with autopsy [9, 10]. Discrepant findings tend to under-estimate luminal stenosis due to circumferential stenosis, eccentric coronary lesions, distal segment, and poor vessel contrast opacification or visualization. Overestimates of plaque may additionally occur due to overlapping vessels, foreshortening, and coronary spasm [9, 10].
Although practical at identifying significant stenoses, angiographer estimation of the precise percent stenosis has been proven to be subject to operator bias and intra- and inter-operator variability (a standard deviation of 18 % luminal stenosis) [11].
Error in the assessment of coronary artery stenosis severity has been demonstrated to be reduced through discussion among multiple observers [11, 12].
Coronary angiography provides detailed anatomic imaging but no functional assessment of myocardial ischemia as with certain other imaging modalities such as stress echocardiography or nuclear imaging. Also, estimation of atherosclerotic lesion stenosis severity requires comparison of the lumen stenosis to an adjacent contrast-filled luminal segment which may have occult plaque not detectable by angiography, thereby underestimating the size of the stenosis of interest.
Quantitative Coronary Angiography (QCA)
The use of digital measurement of luminal narrowing and computer assisted quantitative coronary angiography (QCA) have been shown to increase the accuracy of coronary atherosclerotic plaque measurement (inter-observer standard deviation of percent stenoses 0.9–4.9 %) (Fig. 6.5).
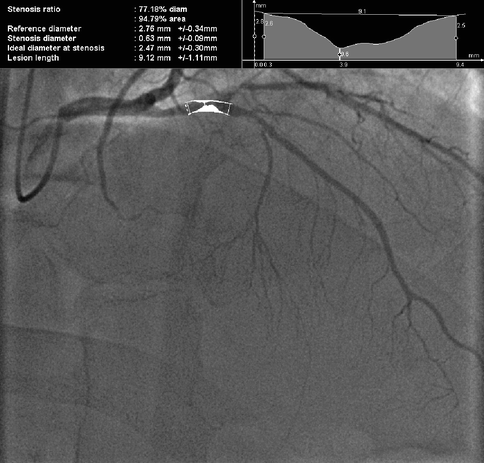
Fig. 6.5
77% diameter (95 % area) stenosis of the proximal left anterior descending (LAD) artery by Quantitative Coronary Angiography (QCA)
QCA may not be routinely employed in everyday practice, except to evaluate stenoses of unclear severity, as its use is time-intensive and most angiographers do not feel that it significantly adds to their trained visual assessment [13].
Patterns of Disease
Angiographic extent of coronary atherosclerosis is, in large part, predicted by the quality of symptoms, particularly the typicality of angina, and clinical risk factors (male gender, family history of coronary artery disease, smoking, elevated blood pressure, high cholesterol, diabetes, physical inactivity) [14–18].
With regard to patient history, patients with definite angina, probable angina, or non-specific chest pain have decreasing rates of angiographic lesions, respectively, and this varies by age [19].
Acute plaque rupture on invasive coronary angiograph is typically visualized as a discrete intraluminal filling defect with defined borders and is largely separated from the adjacent wall. Contrast staining may or may not be present (Fig. 6.6a, b).
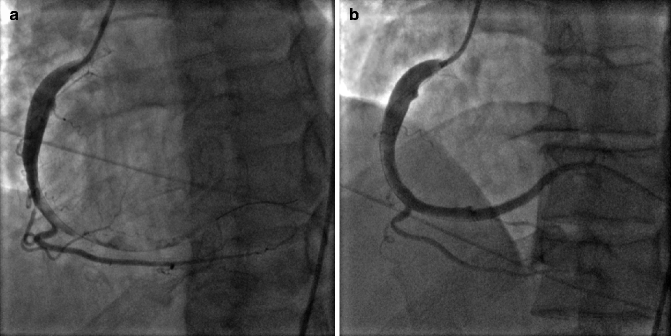
Fig. 6.6
(a) Right coronary artery (RCA) with acute plaque rupture and thrombus from the mid RCA to distal and posterolateral branch. (b) After thrombus aspiration and stenting
Coronary artery dissections appear as a linear lucency and may contain thrombus, and result in reduced distal flow due to compromise of the true lumen. Coronary artery dissections most commonly occur as a complication of percutaneous coronary interventions; however, they rarely may present spontaneously as a rare cause of acute coronary syndrome, especially among peripartum women (Fig. 6.7a, b).
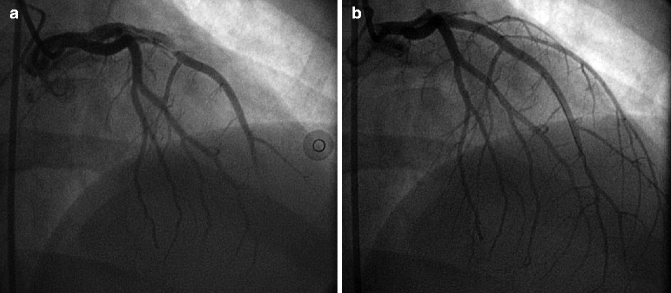
Fig. 6.7
(a) Spontaneous left anterior descending (LAD) coronary artery dissection in a 34 year old postpartum woman who presented with ST-segment elevation myocardial infarction. (b) The lesion was treated with stenting due to failure of medical therapy
Coronary ectasia is often seen on invasive coronary angiography and is defined as a focally irregular and dilated vessel contour. A coronary artery aneurysm is coronary segment > 1.5 times the normal reference vessel diameter (Fig. 6.8a, b).
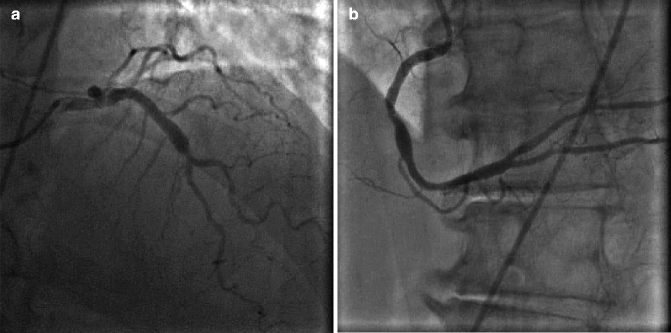
Fig. 6.8
(a) Dilated, ectatic proximal left anterior descending artery. (b) Mid right coronary artery (RCA) aneurysm
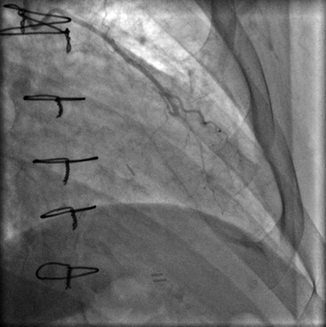
Prior to catheterization, operator knowledge of bypass graft anatomy is very helpful to minimize procedural time and contrast exposure. Some surgeons place coronary artery bypass graft markers to guide future angiographers to the graft ostium:
The left internal mammary artery (LIMA) (Fig. 6.9) is a commonly utilized bypass conduit that originates from left subclavian artery. Its course to the target vessel is often tortuous. It is the most durable of all bypass graft conduits and is typically grafted to LAD, often with a jump segment to prominent native coronary diagonal branch.