© Springer-Verlag Berlin Heidelberg 2013
Hans Geinitz, Mack Roach III and Nicholas van As (eds.)Radiotherapy in Prostate CancerMedical Radiology10.1007/174_2013_915There is Evidence for the Superiority of Protons or Heavy Ions, Contra
(1)
Clinical Oncology Royal Marsden Hospital London, London, SW3 6JJ, UK
(2)
The Royal Marsden Hospital, Fulham Road, London, SW3 6JJ, UK
Abstract
Photon therapy is a safe and effective treatment for localised prostate cancer. It is used widely and has a strong evidence base. For three decades, proponents of proton and heavy ion therapy have claimed that these modalities have theoretical advantages which will translate into clinical benefit. However, there is no current evidence to support this. In this chapter we will assess the theoretical and clinical evidence for proton and heavy ion therapy and compare this with that for photon therapy. The health economic perspective will also be examined. We will show that there is no evidence for the superiority of protons or heavy ions over photon therapy.
1 Introduction
In this chapter we will show that, in the treatment of localised prostate cancer, there is no evidence for the superiority of protons or heavy ions over photon therapy. To set the scene, photon therapy has been successfully used by radiation departments for decades and the evolution from fixed fields to conformal radiotherapy and IMRT (intensity modulated radiotherapy) has resulted in progressive improvements in biochemical control and reduced toxicity (Dearnaley et al. 1999, 2007; Cahlon et al. 2008). Long-term follow-up of patients treated with IMRT has demonstrated excellent biochemical control and acceptable late toxicity (Spratt et al. 2012). Additionally, newer techniques such as image guidance and stereotactic body radiotherapy (SBRT) have the potential to further improve the therapeutic ratio of photon treatments, and produce better outcomes for patients (King et al. 2012). In view of this, photon treatment for localised prostate cancer has set a high standard for alternative modalities to beat.
Those in favour of proton and heavy ion therapy claim that their theoretical advantages (particularly regarding dose distribution) will translate into superior benefit for patients—for example, with reduced late normal tissue toxicity allowing dose escalation. However, notwithstanding over 30 years of research with proton therapy for localised prostate cancer, there is no clinical data to support this assertion and, as is clear from the title of this chapter, we are concerned with evidence for the superiority of proton or heavy ion therapy.
There remains a significant lack of evidence in this field, in that no phase III trials comparing proton or heavy ion therapy with photon therapy exist. As we shall see, there is a good quality phase III trial comparing photons with a higher- and lower-dose proton boost, but this provides little information with regard to the current discussion. Ultimately, phase II studies and retrospective comparisons make up the majority of the data available. Pro-proton groups have argued that a phase III trial of protons versus photons is unnecessary, as the advantages of proton therapy are self-evident (Goitein and Cox 2008). We disagree with this, as will be seen in the coming discussion.
Health economics is also a significant factor here. The setup and running costs of a proton or heavy ion facility are far in excess of even the most advanced photon facility. As such, evidence for superiority of these therapies must be strong before the allocation of resources toward proton or heavy ion therapy can be justified.
In this chapter we will discuss the current theoretical and clinical evidence for proton and heavy ion therapy. Our contention is that these therapies are at very best equivalent to current photon therapy, but at a much greater cost.
2 Theoretical and Planning Considerations
2.1 Physical Properties
The physical properties of proton and heavy ion beams produce steep dose fall-off at depth and at beam margins. Dose can drop from 90 to 10 % over a distance of a few millimetres. Protons and heavy ions are much more sensitive to tissue inhomogeneities than photons and Hounsfield units on computed tomography planning scans must be converted to relative stopping power of each tissue (Joiner and Kogel 2009). A relative biological effectiveness (RBE) of 1.1 is quoted for protons and this value is used by treatment planning programs and when comparing doses to photon therapy (Paganetti et al. 2002). However, RBE may be significantly higher in the terminal part of the proton beam and as much as two to four times higher in the final 2 cm of a heavy ion beam (Kramer et al. 2003). There is therefore significant uncertainty and complexity in planning, compared with photon treatments.
2.2 Organ Motion
It is well established that there is significant variability in the intra- and inter-fraction position of the prostate during radiotherapy treatment. This variability is introduced due to day-to-day differences in rectal, and to a lesser extent bladder, filling. It is also established that this movement can compromise planning target volume (PTV) coverage in photon and proton therapy (Wang et al. 2011). The PTV margin required to account for this motion in photon therapy has been estimated as 6–7 mm in the anterioposterior direction (Wu et al. 2001). Image-guided therapy systems (using implanted fiducial markers) are now in routine use for photon treatments and have had success in reducing PTV and associated rectal and urinary toxicity (Zelefsky et al. 2012b). With regard to proton and heavy ion therapy, given the uncertainties around dose distribution close to the treatment volume (see Sect. 2.1) and the fact that image guidance is not routine, there are significant theoretical concerns regarding toxicity to surrounding tissues. This is particularly the case for patients where the rectal wall is very closely applied to the posterior aspect of the prostate.
2.3 Planning Studies
In an important planning study, Trofimov et al. compared dose distributions for IMRT (using seven equally spaced coplanar fields) and conformal proton therapy (two parallel opposed lateral fields). A dose of 79.2 Gy/GyE was used (Trofimov et al. 2007). This study found that IMRT demonstrated better conformity to the target volume and equivalent rectal dose when compared with conformal proton therapy. Furthermore, the volume of bladder receiving 70 Gy or above was reduced by 34 % with IMRT. Another group compared IMRT with intensity modulated proton therapy (IMPT) (Cella et al. 2001). Both IMRT and IMPT plans used five coplanar fields. For dose up to 99 Gy/GyE conformity to the target volume and organ at risk sparing were equivalent. Thus, in theory, clinical toxicity should be equivalent for IMRT and photon treatments at equivalent doses. If this is the case, there is no scope for dose escalation with proton therapy and no clear theoretical ration for its use.
2.4 Second Malignancy Risk
One advantage of proton therapy and heavy ion therapy over IMRT regularly cited by those advocating these treatments is the theoretical reduction in radiation-induced malignancy risk. IMRT treatments irradiate a significantly larger volume to a low dose (under 30 Gy) when compared with proton treatments. This is the so-called “low dose bath” (Miralbell et al. 2002). Although this increases the theoretical risk of second malignancy, the uncertainty in these calculations may be up to 30 % (Fontenot et al. 2010). A recently published study looking at second malignancy after high-dose IMRT showed an in-field second malignancy risk of 4.8 % at 10 years with a mortality rate of 0.7 % also at 10 years. Just under 900 patients were included. As such, second malignancy may be less relevant to the majority of patients treated for prostate cancer, who are over the age of 70. Additionally, it appears from this data that many second malignancies are treatable or curable (Zelefsky et al. 2012a). There is no clinical evidence comparing second malignancy after photon treatment with that after proton or heavy ion treatment.
3 Review of Clinical Evidence
3.1 Photons Versus Protons or Heavy Ions Alone
3.1.1 Phase III Data and Systematic Reviews
There are currently no reported randomised clinical trials comparing photon treatments with proton or heavy ion therapy alone (De Ruysscher et al. 2012). In localised prostate cancer, multiple systematic reviews of the available clinical data have concluded that, at present, there is no evidence of benefit for proton or heavy ion therapy over photon therapy, with regard to toxicity or tumour control (Brada et al. 2007, 2009; Lodge et al. 2007; Olsen et al. 2007; Allen et al. 2012). In the light of these facts alone it is clear that strong evidence for the superiority of proton or heavy ion therapy to photon therapy is lacking.
3.1.2 Phase II Data
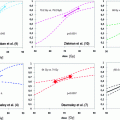
Proponents of proton therapy feel that its potential theoretical advantages, particularly with regard to dose distribution, will translate into a reduction in treatment-related toxicity (Goitein and Cox 2008). If dose-limiting late rectal and urinary toxicity can be reduced with proton therapy, it is claimed that dose escalation could then be undertaken. There is abundant evidence from photon therapy that higher doses of radiation reduce biochemical relapse in the treatment of localised prostate cancer—although a survival benefit has not yet been demonstrated (Viani et al. 2009). Naturally, this improvement comes at the cost of increased late (urinary and rectal) toxicity, and this has limited escalation of dose above 80–90 Gy in 2 Gy fractions, although further clinical benefit is predicted.
However, from the available phase II data, there is no clinical evidence that late toxicity is reduced with proton therapy compared with photon therapy. In fact, in a recent report on the American College of Radiology 03–12 study, 26 % of patients receiving conformal proton monotherapy to a dose of 82 GyE experienced RTOG (Radiation Therapy Oncology Group) grade two or above late toxicity (Coen et al. 2011). This study looked at a cohort of 85 men with a median follow-up of just under 3 years who were treated with a parallel opposed lateral beam technique. It concluded that further proton dose escalation with a conformal technique risked unacceptable late toxicity. A collaborative phase II study by three Japanese proton institutions enrolled 151 patients to be treated with the lower dose of 74 GyE using a conformal parallel opposed beam technique. At 2 years, CTC late grade two or above toxicity was 4.1 and 2.0 % for genitourinary and gastrointestinal symptoms, respectively. These studies can be compared with the Memorial Sloane-Kettering Cancer Centre (MSKCC) review of 170 patients treated with a dose of 81 Gy using a five field IMRT technique (Alicikus et al. 2011). At 10 years of follow-up actuarial prostate-specific antigen (PSA) relapse-free survival was 81, 78, and 62 % for low-, intermediate-, and high-risk disease. Grade two or greater late genitourinary and rectal toxicity was 16 and 3 %, respectively. A further review by the same group of over 1000 patients treated with 86.4 Gy photon IMRT with a median follow-up of 7 years found that grade two or above late genitourinary and rectal toxicity was 21.1 and 4.4 %, respectively (Spratt et al. 2012). The Fox Chase Cancer Centre have also produced published results for IMRT with doses of 74–78 Gy (Eade et al. 2008). A 3 year actuarial grade two and above genitourinary and gastrointestinal toxicities were 3.5 and 2.4 %, respectively.
Turning to therapy with carbon ions, a Japanese group at Chiba University have recently reported on over 700 patients treated with hypofractionated carbon ion therapy for localised prostate cancer (Okada et al. 2012). Doses of 66–57.6 GyE in 20–16 fractions were used. Late toxicities of grade two or above for the 664 patients with at least 1 year of follow-up were 8.0 and 2.4 %, respectively, for genitourinary and gastrointestinal symptoms. A comparison can be made with patients treated in the hypofractionated arms of the multi-centre United Kingdom CHHiP study (Dearnaley et al. 2012). Patients in these arms (150 patients each) received 60 Gy in 20 fractions or 57 Gy in 19 fractions using IMRT. At 2 years RTOG grade two and above toxicity was under 3 % for bladder toxicity and less than 4 % for bowel toxicity.
In summary, although with the caveat that we are comparing between series, it must be concluded that the available phase II evidence does not show an advantage for proton or heavy ion treatments over photon therapy with regard to toxicity. Furthermore, although there are not a large quantity of mature phase II data on proton and heavy ion therapy, it appears that these treatments are at best equivalent to photon therapy in terms of tumour control (Jensen et al. 2011). These factors argue against the possibility of significant dose escalation with protons or heavy ions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree