■
Introduction: Using Cardiovascular Imaging
Cardiovascular diseases remain one of the most common causes of death in industrialized nations. Over the last decade, substantial technical advances in cardiovascular imaging have been made and have helped mature such modalities from research techniques into clinical applications. Ischemic cardiac disease is defined as an efficiency loss of the myocardium due to inadequate oxygen supply, which is mostly caused by obstructive coronary artery disease (CAD). Several parameters that can be obtained through cardiovascular imaging have been shown to allow for more precise risk stratification and management in patients with ischemic heart disease.
Indications for Imaging
Risk assessment remains the main indication for cardiovascular imaging in clinical routine. Coronary computed tomography angiography (CCTA) has been incorporated into most major guidelines as a first-choice standard examination to detect CAD, assess coronary artery bypass graft (CABG) lumen patency, and visualize congenital heart disease. A number of multicenter studies have demonstrated high sensitivity and specificity for CCTA in comparison with invasive coronary angiography (ICA). Although the severity of coronary stenosis may be overestimated with CCTA, its high negative predictive value has established it as a modality to rule out CAD reliably. The use of CCTA in the emergency department to triage patients with acute chest pain and suspected CAD has also been recommended by multiple studies.
Although CCTA is per definition an anatomic examination, other functional imaging modalities generally need to be used to assess the hemodynamic significance of detected coronary stenosis. Such myocardial perfusion imaging (MPI) is mostly performed using single-photon emission CT (SPECT) or magnetic resonance imaging (MRI), although other modalities can also be used (e.g., positron emission tomography, stress echocardiography, fractional flow reserve CT [CT-FFR]). This explains why a comprehensive workup in patients with ischemic cardiac disease is commonly based on multimodality imaging. However, multiple factors and patient characteristics influence the selection of an appropriate imaging modality and protocol ( Box 30.1 ).
Technical Parameters
CT detector design and arrays
Available CT radiation and contrast dose reduction techniques
Postprocessing algorithms (3D, 4D, and CT-FFR)
MR field strength and bore width
Patient Factors
Age
Gender
Body weight and habitus
Heart rate
Heart rhythm
Blood pressure
Medical and surgical history
Renal function
Ability to follow breathing commands
Presence of devices and implants
Claustrophobia
Clinical Parameters
Indications for imaging
Appropriateness for imaging
Specific clinical questions
Impact on further clinical pathway
Determining the Appropriate Imaging Modality
X-Ray (Chest X-Ray, Invasive Coronary Angiography)
In most patients presenting to the emergency department, an initial chest x-ray is obtained. Although not routinely used to detect ischemic cardiac disease, it can provide certain indicators of CAD because extensive coronary calcification may be visible or characteristic enlargement of certain cardiac chambers may indicate valvular disease and heart failure.
In routine clinical practice assessment of ischemic cardiac disease, x-ray technology is usually used more during ICA. This invasive approach remains the gold standard to assess CAD, especially because FFR measurements also allow for an accurate evaluation of the hemodynamic significance of coronary stenosis. CAD detected on CCTA may be reaffirmed using ICA, the severity can be quantified using FFR, and it can be simultaneously treated with balloon angioplasty and stent placement when appropriate.
Computed Tomography (Coronary CT Angiography, CT Myocardial Perfusion Imaging)
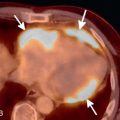
Due to several major technical improvements over the last decades, CT has evolved into a routinely used modality to detect multiple cardiac pathologies. Currently, it is still mostly used to detect or rule out CAD. For an initial evaluation, a low-dose, unenhanced calcium scoring scan is usually performed to detect calcified coronary plaques ( Fig. 30.1 ). The resulting Agatston score based on this dataset can be used for risk stratification based on an extensive body of research regarding the prognostic value of this calcium score. Nevertheless, especially in younger patients, noncalcified coronary soft plaque or, in the acute setting, a clot or embolus may be present, which would remain undetected on a noncontrast scan. Therefore, a contrast-enhanced CCTA study may be necessary to assess coronary artery patency and high-risk coronary anomalies (e.g., interarterial or intramural course). CCTA is also used to evaluate CABG patency, and newer scanner generations allow for the detection of in-stent thrombosis due to higher spatial and temporal resolution and improved metal artifact reduction algorithms. The combination of recently introduced dose-saving technologies has allowed for a substantial dose reduction, down to submillisievert levels in clinical practice. Thus CCTA has been established as an adequate first-line, rule-out test in patients with suspected CAD, with low to intermediate probability.

In an effort to provide comprehensive cardiac assessment, static and dynamic MPI using CT (CTMPI) has been made feasible ( Fig. 30.2 ). A growing body of evidence demonstrates that additional CTMPI may be helpful to identify patients with hemodynamically relevant coronary artery stenosis. An advantage of CTMPI over other established myocardial viability imaging modalities is the opportunity for a comprehensive, single-modality cardiac assessment. By performing both examinations in the same time slot, without additional patient positioning, this may ultimately prove to be a more time-efficient and cost-effective imaging solution.


Cardiac Magnetic Resonance
Due to several major technical improvements over the last decades, cardiac magnetic resonance (CMR) has evolved into the gold standard for the assessment of left and right ventricular function and myocardial viability without the use of ionizing radiation. There is growing evidence that stress myocardial perfusion CMR is a superior alternative to SPECT for the detection of perfusion deficits. Some have even suggested that this modality may also be particularly useful in women, whose epicardial coronary arteries show no obstruction but who have suspected coronary microvascular dysfunction. An increasing number of pacemakers and intravascular stents are MR-compatible, and several single-shot, free-breathing protocols and motion correction algorithms are available for most imaging indications. In addition, CMR late gadolinium enhancement (LGE) imaging remains the examination of choice for the visualization of myocardial necrosis and scar, as well as the myocardial involvement in a variety of other structural diseases (e.g., cardiac amyloidosis, sarcoidosis, myocarditis). Furthermore, stress dobutamine CMR is a viable alternative to stress dobutamine echocardiography.
Nevertheless, CMR still tends to be more time-consuming than cardiac CT, and some patients still refuse or abort MR examinations due to severe claustrophobia. Thus some of the concepts of CMR, including late-enhancement imaging, have been transferred to CT to allow for similar examination characteristics in selected patients, although such techniques are still under development.
Nuclear Studies
SPECT remains an established MPI technique to detect myocardial ischemia due to the substantial body of research regarding its value as a prognostic indicator. Nevertheless, its relatively high radiation exposure, requirement for a radionuclide tracer, and rather low specificity have led to the development of the other aforementioned MPI techniques. Although cardiac positron emission tomography using 82 Rb or 13 N-ammonia is also feasible, it is only used by a minority in clinical routine, primarily due to the logistical difficulties in obtaining these radiotracers.
■
CT Imaging Algorithms
Examining Patients With Suspected Coronary Artery Disease
When a patient is referred for cardiac imaging, the radiologist should tailor an appropriate imaging protocol determined by indication, the patient’s clinical parameters, and the technical capabilities of the scanner used. Coronary artery imaging currently remains mainly a CT domain. First, it should be determined whether the clinical question can be answered with purely anatomic data of the coronary arteries or whether it requires functional data and possibly even additional myocardial perfusion assessment. In addition, in the emergency room setting, an emergent triple rule-out CTA of the entire chest may be warranted, instead of standard CCTA.
Patients with a low to intermediate pretest likelihood for CAD are the ideal candidates for CCTA. Some patients with known CAD may benefit from a CCTA—for example, to assess for coronary stent or bypass graft patency. Patients with a prior CABG require a larger field of view to visualize the internal mammary arteries fully.
A noncontrast, low-dose, calcium scoring scan is usually performed for initial risk assessment, for an initial overview of cardiac anatomy, and for optimal protocol selection, but can be omitted in patients with implanted coronary stents, prior infarct, or a CABG.
Image quality is mainly determined by the used acquisition protocol and the patient’s heart rate and body habitus. Most cardiac CT examinations require electrocardiogram (ECG) synchronization, even though newer scan technologies may provide exemptions. A widely accepted guideline is to scan patients with a heart rate <80 beats/min with prospectively ECG-triggered protocols to reduce radiation exposure, whereas patients with a heart rate of >80 beats/min are scanned with retrospectively ECG-gated protocols to ensure diagnostic image quality.
To achieve a lower heart rate, some patients may require oral or IV beta blockers unless contraindicated (e.g., in severe asthma). Many patients referred for cardiac CT also have a history of hypertension or arrhythmia and are already medicated with beta blockers. Sublingual application of nitroglycerin provides coronary artery dilation, which may improve visualization. It should be administered 3 to 5 minutes prior to the scan but is contraindicated in patients currently medicated with other vasodilators (e.g., sildenafil) because severe hypotension is a possible complication.
Both bolus tracking and test bolus techniques can be used for CCTA to ensure optimal scan timing. Depending on the scanner generation, functional analysis requires retrospectively ECG-gated protocols or can be performed using newer padding techniques for prospectively triggered acquisition modes. Late-enhancement imaging 7 to 15 minutes after the angiographic phase to detect myocardial scarring can be optionally performed, depending on the patient’s clinical history and indications for imaging.
Image reconstruction and three-dimensional postprocessing plays a crucial role in cardiac CT. Patients with severe calcifications or stents may benefit from reconstructions with sharper kernels, which could improve vessel conspicuity. Curved multiplanar reconstructions (cMPRs) are helpful for the visualization of plaque geometry and for the assessment of stenosis severity, particularly in complex cases. Alternatively, where available, CT-derived FFR values could be assessed and included in the clinical decision making process. Three-dimensional volume rendering is especially useful for visualization of CABG anatomy and cardiac malformations. Snapshot images should be made available for colleagues from other subspecialties for intervention planning. It should be noted, however, that the severity of coronary artery stenosis is usually slightly overestimated on CCTA compared to ICA.
Reporting the Findings
Each cardiac CT report should begin with describing the scanning technique and image reconstruction parameters. If available, the resulting Agatston score from the calcium scoring scan should be stated with the corresponding MESA (Multi-Ethnic Study of Atherosclerosis) percentile adjusted for age, race, and gender ( http://www.mesa-nhlbi.org/calcium/input.aspx ). For analysis of CCTA images, it is usually helpful to provide a brief description for each major coronary artery (right, left main, left anterior descending, left circumflex coronary arteries, and, if present, the ramus intermedius) describing vessel course, plaque presence, and distribution. Due to our inability to quantify stenosis severity accurately, coronary stenosis should be described qualitatively as mild (<30%), moderate (30%–70%), or severe (>70%). Coronary anomalies, especially those with malignant potential, should also be described here.
Even if normal, cardiac anatomy should be mentioned at least briefly, and anatomy of the greater vessels should be assessed. Special attention should be paid to the myocardium because perfusion defects (acute or chronic) may be visible as segments with hypoattenuation. Chronic changes, including myocardial thinning, fatty metaplasia, calcifications, and aneurysm, should also be reported. If functional imaging was performed, quantitative results (e.g., ejection fraction, end-diastolic and end-systolic volumes) should be reported, and regional wall motion abnormalities should be mentioned, when present.
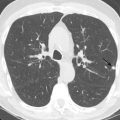
It is imperative to evaluate all provided image series for extracardiac findings because CCTA may be the first cross-sectional imaging modality performed in patients with suspected CAD without any prior history of hospitalization. The lungs should be examined in a separate image series reconstructed with a wide field of view and sharper lung kernel to avoid missing smaller pulmonary nodules. Acute or chronic pulmonary disease (e.g., pneumonia, emphysema, fibrosis) and pleural effusion should also be described. A separate image series of the thorax reconstructed with a dedicated soft tissue kernel should be reviewed to detect masses or lymphadenopathy. Finally, the chest wall, osseous structures, and partially visualized abdomen (and, in some cases, the lower neck) should be examined.
The radiologist, unfortunately, often has only little knowledge regarding the scheduled clinical workflow of the patient after CCTA. In addition, the interpretation of a CCTA report by the treating physician may vary from the radiologist’s report but also has the potential to alter the clinical pathway substantially. CCTA is an excellent examination for triage and to rule out CAD, but lacks specificity to predict actual hemodynamic relevance of stenosis. Therefore it is advisable to give a broad description of the coronary status and to highlight potentially significant stenosis, which may represent culprit lesions without stating the actual percentage of stenosis values. Suggestions for additional imaging procedures (CMR, SPECT) may be appreciated by the treating physician but are not mandatory. In conclusion, as with all reports, the main clinical question should be answered, instead of reusing the same report template for all cases.
Reducing Radiation and Contrast Dose
Several recent technologic innovations have helped substantially lower radiation exposure from CCTA and required contrast material dose in routine clinical practice and can be applied in most patients. Conventionally, CCTA has been performed using retrospectively ECG-gated protocols with continuous radiation and relatively high tube voltages for sufficient tissue penetration, with effective doses of 10 to 18 mSv. Prospectively ECG-gated protocols result in intermittent radiation and consequently lower radiation exposure substantially. Depending on the patient’s heart rate and scanner generation, patients can be examined using these prospectively-gated protocols in sequential (<80 beats/min) or high-pitch (<65 beats/min) acquisition. High-pitch scanning has made CCTA with <1 mSv feasible, but remains restricted to specific patient populations.
Newer scanner generations provide an automated in-scan adaptation of tube voltage and tube current modulation, which can substantially reduce radiation exposure without impacting image quality. Although older scanners were operated with a tube voltage of 120 kVp, newer scanners allow for a reference tube voltage of as low as 70 kVp due to improvements in detector technology ( Fig. 30.3 ). Low tube voltage acquisition during contrast-enhanced CT allows for a significant dose reduction and may also result in a superior contrast-to-noise ratio due to a far stronger iodine attenuation compared to standard acquisition, with only a moderate increase in image noise. In addition, postprocessing using iterative reconstruction algorithms can mitigate such distinct image noise, further improving image quality with low tube voltage acquisition.
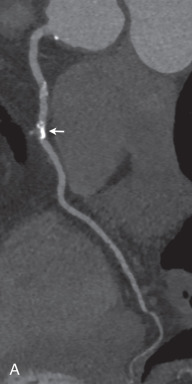



Most of these techniques for the reduction of radiation exposure can be simultaneously applied for reducing contrast material volume. Due to the increased iodine attenuation with low tube voltage acquisition, less contrast material is required. In addition, high-pitch CCTA protocols usually require less contrast material also due to rapid image acquisition.
■
Cardiac Magnetic Resonance Imaging Algorithms
Contraindications to the Use of Cardiac Magnetic Resonance
Due to the strong magnetic field used to operate the MR scanner, there are certain safety provisions for which every patient undergoing CMR must be screened. Unknown active devices, ferromagnetic implants, unknown aneurysm clips, metallic fragments in the eye, implantable cardioverter-defibrillators (ICDs), and any implant known to be MR-unsafe are absolute contraindications for CMR. There are relative contraindications when the CMR study can possibly be performed after a thorough risk-benefit assessment. Such conditions include severe claustrophobia, large metal wires, and active devices that have not been tested for MR safety. Most pacemakers belong to the latter group; pacemakers, however, can be programmed to MR mode before the CMR examination after consultation with the treating cardiologist. When scanning these patients, the imaging parameters have to be adjusted using more conventional gradient echo sequences to reduce energy deposition and metal-induced susceptibility artifacts.
The presence of newer stents, heart valves, coils, and occluder devices represents relative contraindications. Patients with such implants can usually be imaged after a certain time specified by the manufacturer. Novel MR-conditional implantable pacemakers are now available, and new generations of ICDs have been tested for MR use. Although an increasing number of devices and implants released from year to year are MR-safe, the artifacts caused by the presence of these implants still represent a challenge in image interpretation. However, because most pacemaker and ICD leads are anchored in the right heart, the visualization of the left ventricle is only minimally affected in most cases.
Examining Patients With Suspected Coronary Artery Disease
Although CT is the current noninvasive standard of care for the evaluation of coronary artery morphology, and there are efforts to develop CT techniques for the assessment of functional parameters, the evaluation of myocardial function and perfusion remains within the domain of CMR, mainly due to its significantly higher temporal resolution. When a patient with CAD is referred for CMR, a variety of diagnostic questions may arise to determine the imaging protocol suitable for the given clinical scenario.
Patients with heart failure of unknown cause and patients with known CAD typically undergo a routine protocol, sometimes referred to as a viability protocol, which includes localizers, T2-weighted, cine, and rest perfusion images, as well as early and late postcontrast inversion recovery images. The acquisitions are ECG-gated. Most of these images are segmented—that is, they are collected in several consecutive heartbeats, which require 7- to 20-s long breath-holds to keep the diaphragm at a relatively constant level. Some of the images are single shot, meaning that they are acquired within a single heartbeat.
Cine loops are acquired with 20- to 40-ms temporal resolution in three standard long-axis (two-, three-, and four-chamber views) and multislice short-axis orientations, allowing for the assessment of global and regional cardiac function and valves. Rest myocardial perfusion is assessed after the administration of the gadolinium-based contrast agent, which facilitates the evaluation of the myocardial first-pass contrast uptake. Rest perfusion is especially beneficial in cases of a suspected intracardiac thrombus or mass. Early gadolinium enhancement images are collected 2 minutes postcontrast to assess for thrombus or microvascular perfusion defects, and LGE imaging is performed 10 to 20 minutes after contrast administration to evaluate myocardial contrast accumulation.
In patients with symptoms suggesting stress-inducible ischemia, a stress protocol can be added to the above-described routine protocol. Dobutamine stress CMR can be performed to detect stress-induced wall motion abnormalities. However, the administration of dobutamine is contraindicated in several cardiac conditions, such as severe arterial hypertension, unstable angina, acute myocardial infarction, severe aortic stenosis, and hypertrophic obstructive cardiomyopathy. A more frequently performed protocol is vasodilator stress perfusion imaging, designed to detect stress-induced ischemia in the myocardium. This approach is based on the considerably slower first-pass kinetics of the contrast agents in the myocardial territories affected by ischemia. Adenosine (0.14 mg/kg/min for 3 minutes) and regadenoson (0.4-mg bolus) are the most frequently used pharmacologic stressors. One day before the administration of the stressor, food and beverages containing caffeine must be suspended. The use of these stressors is contraindicated in several conditions such as hypersensitivity to the medication, second- or third-degree atrioventricular block, long QT syndrome, sick sinus syndrome, active wheezing in chronic obstructive pulmonary disease, severe hypotension, and unstable angina. Heart rate, oxygen saturation, and blood pressure should be monitored before, during, and after the stress protocol.
CAD is often associated with valvular disease (e.g., aortic valve calcification and stenosis, mitral insufficiency due to papillary muscle infarction). In such cases, CMR offers the opportunity to evaluate not only valve anatomy and motion, but also the blood flow through the valve. Phase contrast velocity mapping sequences are used to map blood velocity, similarly to Doppler echocardiography. Using the phase contrast acquisition, peak velocities as well as the forward and regurgitant volumes can be quantitatively assessed. Novel phase contrast CMR sequences can even be applied in a free-breathing fashion and in four dimensions.
Coronary MR angiography is a relatively new domain of CMR in the clinical setting. Several experimental studies have been conducted to investigate the feasibility of CMR for the visualization of the coronary arteries. Although CMR outperforms CT in temporal resolution, its spatial resolution is still inferior to CT. In addition, the collection of a three-dimensional dataset requires imaging during several heartbeats, resulting in alignment issues between images acquired at different heartbeats and multiple breath-holds. There are some prototype sequences available implementing combined ECG and respiratory gating or various motion correction algorithms to provide good image quality and also patient comfort by eliminating breath-holds. Using such sequences, the proximal and mid courses of the coronary arteries can be visualized. However, the presence of calcification in the coronary arteries (and in the aortic valve) leads to further challenges in image interpretation due to significant dephasing artifacts.
Administering a Gadolinium Agent
Gadolinium-based contrast agents are relatively safe. The number of hypersensitivity reactions reported is significantly less than for iodinated contrast media. A major concern regarding these agents arose several years ago, when cases with nephrogenic systemic fibrosis (NSF) were associated with the administration of certain gadolinium agents. However, the causal relationship between gadolinium administration and the development of NSF has not been clarified so far. Newer concerns have been recently voiced over the long-term permanent accumulation of gadolinium in the body, but the exact clinical significance is yet unknown. Novel contrast agents with a cyclic structure exhibit significantly higher molecular stability, providing increased patient safety. According to the current guidelines, a gadolinium agent must not be used in patients with severe kidney problems (glomerular filtration rate [GFR] <30 mL/min per 1.73 m 2 ) and in newborns younger than 4 weeks of age.
Reporting the Findings
The report should be started with the description of the CMR protocol and the contrast medium and amount used, followed by the findings in a structured order. A general description of the calibers of the atria and ventricles, interatrial and interventricular septal discontinuity, and pericardial status should be provided.
Further assessment of the CMR images requires a complex overview of the various acquisitions. It is particularly important to match the findings of the T2-weighted, cine, perfusion, and early and late enhancement images. For example, a signal increase in a certain myocardial territory in T2-weighted images may correspond to hypokinesis of the same segment in cine, a hypointense region on rest perfusion and early gadolinium enhancement images, and hyperintensity in the delayed phase, consistent with acute myocardial infarction. Thus the findings in each image set should be separately reported, matched, and summarized as an impression.
The presence of myocardial edema in T2-weighted images should be discussed. T2 hyperintensity may indicate myocardial edema ( Fig. 30.4 ), whereas T2 hypointensity may represent the presence of heme-containing blood degradation products due to intramyocardial hemorrhage. Edema may indicate acute infarction or inflammation. Hemorrhage may be caused by the revascularization of an occluded coronary artery.