GROSS ANATOMY
Overview
- •
Kidneys are paired, bean-shaped, retroperitoneal organs
- ○
Function
- –
Removal of excess water, salts, and wastes of protein metabolism from blood
- –
Regulation of water and electrolyte balance
- –
Secretion of hormones that control blood pressure, bone and blood production
- –
- ○
Anatomic Relationships
- •
Located in retroperitoneum, within perirenal space, surrounded by renal fascia (of Gerota)
- •
Each adult kidney is ~ 9-14 cm in length, 5 cm in width, 3 cm in thickness
- •
Both kidneys lie on quadratus lumborum muscles, lateral to psoas muscles, between T12-L3
Internal Structures
- •
Kidneys are hollow centrally with renal sinus occupied by fat, renal pelvis, calyces, vessels, and nerves
- •
Renal hilum: Concavity where artery enters and vein and ureter leave renal sinus
- •
Renal pelvis: Funnel-shaped expansion of upper end of ureter
- ○
Receives major calyces (infundibula) (2 or 3), each of which receives minor calyces (2-4)
- ○
- •
Renal papilla: Pointed apex of renal pyramid of collecting tubules that excrete urine
- ○
Each papilla indents a minor calyx
- ○
7-10 papilla per kidney
- ○
- •
Renal cortex: Outer part, contains renal corpuscles (glomeruli, vessels), proximal portions of collecting tubules and loop of Henle
- •
Renal medulla: Inner part, contains renal pyramids, distal parts of collecting tubules, and loops of Henle
- •
Vessels, nerves, and lymphatics
- ○
Artery
- –
Usually 1 for each kidney
- –
Arise from aorta at about L1-L2 vertebral level
- –
- ○
Vein
- –
Usually 1 for each kidney
- –
Lies in front of renal artery and renal pelvis
- –
- ○
Nerves
- –
Autonomic from renal and aorticorenal ganglia and plexus
- –
- ○
Lymphatics
- –
To lumbar (aortic and caval) nodes
- –
- ○
IMAGING ANATOMY
Overview
- •
Ultrasound is 1st-line modality for acute or chronic disease, flank pain, and suspected complications of acute pyelonephritis
Internal Contents
- •
Renal capsule
- ○
Normal kidneys are well-defined due to presence of renal capsule and are less reflective than surrounding fat
- ○
- •
Renal cortex
- ○
Renal cortex has reflectivity that is less than adjacent liver or spleen
- ○
If renal cortex brighter than normal liver (hyperechoic), high suspicion of renal parenchymal disease
- ○
- •
Medullary pyramids
- ○
Medullary pyramids are less reflective than renal cortex
- ○
- •
Corticomedullary differentiation
- ○
Margin between cortex and pyramids is usually well-defined in normal kidneys
- ○
Margin between cortex and pyramids may be lost in presence of generalized parenchymal inflammation or edema
- ○
- •
Renal sinus
- ○
Echogenic due to the fat that surrounds blood vessels and collecting systems
- ○
Outline of renal sinus is variable, smooth to irregular
- ○
Renal sinus fat may increase in obesity, steroid use, and sinus lipomatosis
- ○
Renal sinus fat may decrease in cachectic patients and neonates
- ○
If sinus echoes are indistinct in noncachectic patient, tumor infiltration or edema should be considered
- ○
- •
Collecting system (renal pelvis and calyces)
- ○
Not usually visible in dehydrated patient
- –
AP diameter of renal pelvis in adults should be < 10 mm
- –
- ○
May be seen as physiological “splitting” of renal sinus echoes in patients with a full bladder undergoing diuresis
- ○
Possible obstruction can be excluded by performing postmicturition images of collecting system and looking for ureteral jets in the bladder with color Doppler
- ○
Physiological “splitting” of renal sinus echoes is common in pregnancy
- –
Causes of dilatation of pelvicalyceal system include mechanical obstruction by enlarging uterus, hormonal factors, increased blood flow, and parenchymal hypertrophy
- –
May occur as early as 12 weeks into pregnancy
- –
Seen in up to 75% of right kidneys at 20 weeks into pregnancy, less common on left side, thought to be due to cushioning of ureter from gravid uterus by sigmoid colon
- –
Obvious dilatation of pelvicalyceal system can be seen in 2/3 of patients at 36 weeks
- –
Changes usually resolve within 48 hours after delivery
- –
- ○
- •
Renal arteries
- ○
Normal caliber 5-8 mm
- ○
2/3 of kidneys are supplied by single renal artery arising from aorta
- ○
1/3 of kidneys are supplied by 2 or more renal arteries arising from aorta
- –
Main renal artery may be duplicated
- –
Accessory renal arteries may arise from aorta superior or inferior to main renal artery
- –
Accessory renal arteries enter kidney in hilum or at poles
- –
Extrahilar accessory renal arteries may arise from ipsilateral renal artery, ipsilateral iliac artery, aorta, or retroperitoneal arteries
- –
- ○
Spectral Doppler
- –
Open systolic window, rapid systolic upstroke occasionally followed by secondary slower rise to peak systole with subsequent diastolic delay but persistent forward flow in diastole
- –
Continuous diastolic flow is present due to low resistance in renal vascular bed
- –
Low-resistance flow pattern is also present in intrarenal branches
- –
Normal peak systolic velocity (PSV) 75-125 cm/s, not more than 180 cm/s
- □
> 200 cm/s is abnormal
- □
- –
Resistive index (RI) is (peak systolic velocity – end diastolic velocity)/peak systolic velocity; normal < 0.7
- –
Pulsatility index (PI) is (peak systolic velocity – end diastole velocity)/mean velocity, normal < 1.8
- –
- ○
- •
Renal veins
- ○
Normal caliber 4-9 mm
- ○
Formed from tributaries that coalesce at renal hilum
- ○
Right renal vein is relatively short and drains directly into IVC
- ○
Left renal vein receives left adrenal vein from above and left gonadal vein from below
- ○
Left renal vein crosses midline between aorta and superior mesenteric artery
- ○
Spectral Doppler
- –
Normal PSV 18-33 cm/s
- –
Spectral Doppler in right renal vein mirrors pulsatility in IVC
- –
Spectral Doppler in left renal vein may show only slight variability of velocities consequent upon cardiac and respiratory activity
- –
- ○
Size
- •
Bipolar length is found by rotating transducer around its vertical axis such that the longest craniocaudal length can be identified
- •
Normal size between 10-15 cm
- •
Volume measurements
- ○
May be more accurate but is time consuming
- ○
3D ellipsoidal formula can be used for volume estimation
- –
Length x AP diameter x transverse diameter x 0.5
- –
- ○
Consistency and changes in volume over time more important
- ○
ANATOMY IMAGING ISSUES
Imaging Recommendations
- •
Right kidney
- ○
Liver used as acoustic window
- ○
Transducer placed in subcostal or intercostal position
- ○
Varying degree of respiration is useful
- ○
Raising patient’s right side and scanning laterally/posterolaterally may be useful
- ○
- •
Left kidney
- ○
More difficult to visualize due to bowel gas from small bowel and splenic flexure
- ○
Usually easier to search for left kidney using posterolateral approach with left side raised
- ○
Full right lateral decubitus with pillow under right flank and left arm extended above head may be useful in difficult cases
- –
Spleen can be used as acoustic window for imaging upper pole of left kidney
- –
- ○
- •
Posterior approach for both kidneys
- ○
Useful for interventional procedures such as renal biopsy, nephrostomy
- ○
Use bolster or pillow under the patient’s abdomen to decrease lordosis
- ○
Image quality may be impaired by thick paraspinal muscles and ribs shadowing
- ○
- •
Renal arteries
- ○
Origins best seen from midline anterior approach
- ○
Right renal artery can usually be followed from origin to kidney
- ○
Left renal artery often requires posterolateral coronal transducer scanning position for visualization
- ○
- •
Renal veins
- ○
Best seen on transverse scan from anterior approach
- ○
May also be seen on coronal scan from posterolateral coronal
- ○
- •
Use highest frequency transducer appropriate for patient body habitus: 2-9 MHz curvilinear or 8-12 MHz linear
- •
Compound and harmonic techniques to decrease artifacts
- •
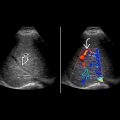
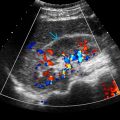
Color Doppler for global renal perfusion, presence of flow in lesions, segmental hypoperfusion in acute pyelonephritis/infarcts and bladder jets
- •
Spectral Doppler: Renal artery stenosis, arteriovenous fistula
Key Concepts
- •
Accessory renal vessels
- ○
Accurate diagnosis necessary when planning surgery (e.g., resection, transplantation)
- ○
Due to limitations of ultrasound, CT arteriography, magnetic resonance angiography, or digital subtraction angiography are more sensitive and accurate
- ○
- •
Normal variants may mimic disease
- ○
Dromedary hump and hypertrophied column of Bertin may be mistaken for renal tumors
- ○
- •
Congenital anomalies very common
- ○
Leading cause of renal failure in children
- ○
Early diagnosis important
- ○
EMBRYOLOGY
Embryologic Events
- •
Congenital structural anomalies include abnormal renal number, position, structure, and vessels
- ○
Abnormal number: Absence of 1 or both kidneys; supernumerary kidney
- ○
Abnormal position: Pelvic kidney, crossed-fused renal ectopia, malrotation, ptosis
- ○
Abnormal structure
- –
Duplication: Results from lack of fusion and commonly produces an enlarged kidney with 2 separate hila and pelvicalyceal systems, these may join or continue as 2 ureters
- □
Ureters may be completely separate until they join the bladder or join proximal to the bladder
- □
“Duplex kidney”: Bifid renal pelvis with single ureter
- □
- –
Hypertrophied column of Bertin (lobar dysmorphism; fetal lobulation; hilar lip)
- –
Pelviureteric junction obstruction
- –
- ○
Often accompanied by anomalies of other systems
- –
VATER acronym: Vertebral, anorectal, tracheoesophageal, radial ray, renal anomalies
- –
- ○
RENAL FASCIA AND PERIRENAL SPACE


KIDNEYS IN SITU


RENAL ARTERY

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree